LEADER Senna Plus Stool Softener Laxative SOFTGEL
LEADER Senna Plus Stool Softener Laxative SOFTGELS
6efe36a9-051d-435e-8965-9339bb41bf33
HUMAN OTC DRUG LABEL
Sep 3, 2025
Cardinal Health 110, LLC. DBA Leader
DUNS: 063997360
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Docusate sodium, Sennosides
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (14)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Packaging
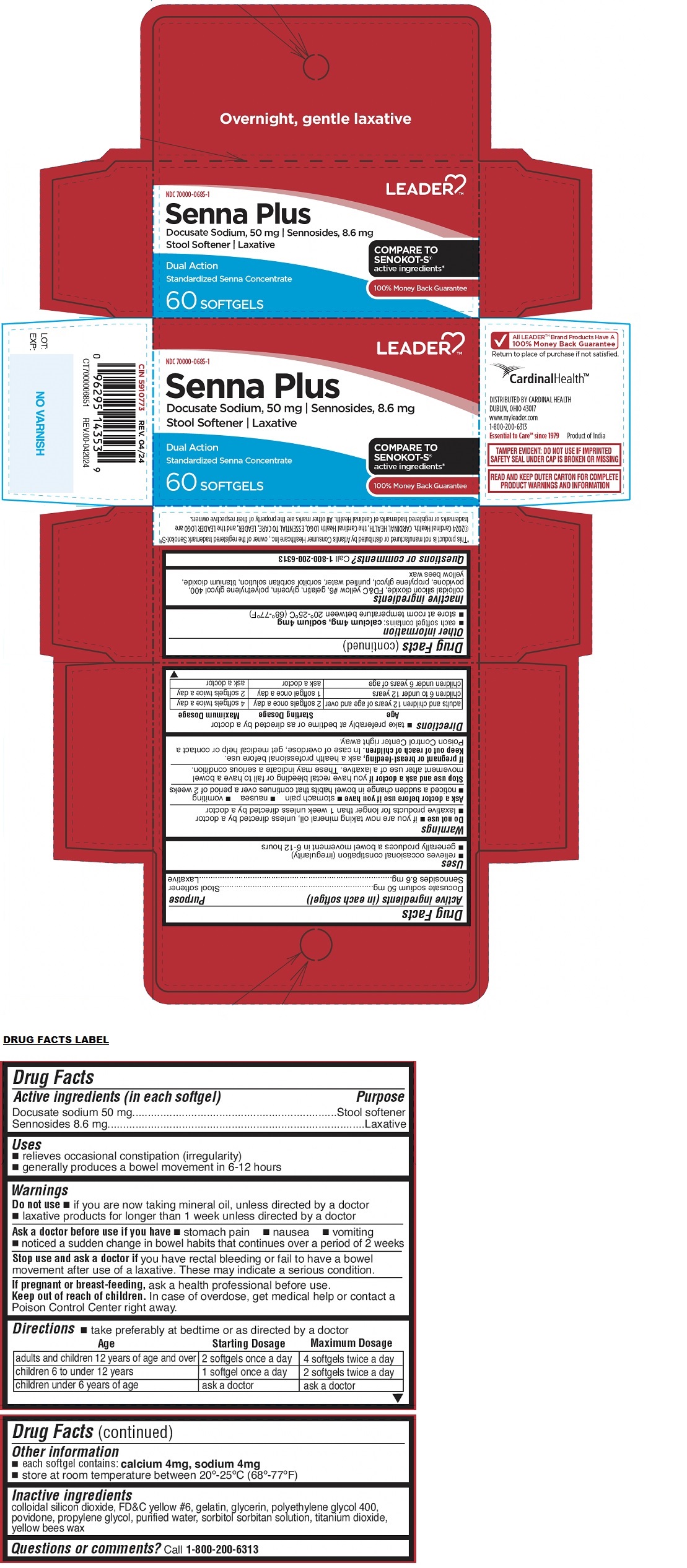
INDICATIONS & USAGE SECTION
Uses
• relieves occasional constipation (irregularity)
• generally produces a bowel movement in 6-12 hours
OTC - ACTIVE INGREDIENT SECTION
Active ingredients (in each softgel)
Docusate sodium 50 mg
Sennosides 8.6 mg
OTC - PURPOSE SECTION
Purpose
Stool softener
Laxative
SPL UNCLASSIFIED SECTION
COMPARE TO SENOKOT-S**®**** active ingredients***
Dual Action
Standardized Senna Concentrate
All LEADER™ Brand Products Have A100% Money Back Guarantee
Return to place of purchase if not satisfied.
CardinalHealth™
DISTRIBUTED BY CARDINAL HEALTH
DUBLIN, OHIO 43017
www.myleader.com
1-800-200-6313
Essential to Care™ Since 1979
Product of India
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
READ AND KEEP OUTER CARTON FOR COMPLETE PRODUCT WARNINGS AND INFORMATION
Overnight, gentle laxative
*This product is not manufactured or distributed by Atlantis Consumer Healthcare Inc., owner of the registered trademark Senokot-S ®
©2024 Cardinal Health. CARDINAL HEALTH, the Cardinal Health LOGO, ESSENTIAL TO CARE, LEADER, and the LEADER LOGO are trademarks or registered trademarks of Cardinal Health. All other marks are the property of their respective owners.
WARNINGS SECTION
Warnings
Do not use• if you are now taking mineral oil, unless directed by a doctor • laxative products for longer than 1 week unless directed by a doctor
Ask a doctor before use if you have• stomach pain • nausea • vomiting • noticed a sudden change in bowel habits that continues over a period of 2 weeks
Stop use and ask a doctor ifyou have rectal bleeding or fail to have a bowel movement after use of a laxative. These may indicate a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
• take preferably at bedtime or as directed by a doctor
|
Age |
Starting Dosage |
Maximum Dosage |
|
adults and children 12 years of age and over |
2 softgels once a day |
4 softgels twice a day |
|
children 6 to under 12 years |
1 softgel once a day |
2 softgels twice a day |
|
children under 6 years of age |
ask a doctor |
ask a doctor |
STORAGE AND HANDLING SECTION
Other information
• each softgel contains:calcium 4 mg, sodium 4 mg
• store at room temperature between 20°-25°C (68°-77°F)
INACTIVE INGREDIENT SECTION
Inactive ingredients
colloidal silicon dioxide, FD&C yellow #6, gelatin, glycerin, polyethylene glycol 400, povidone, propylene glycol, purified water, sorbitol sorbitan solution, titanium dioxide, yellow bees wax
OTC - QUESTIONS SECTION
Questions or comments?
Call1-800-200-6313
