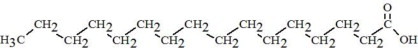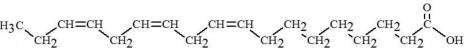Smoflipid
These highlights do not include all the information needed to use SMOFLIPID safely and effectively. See full prescribing information for SMOFLIPID. SMOFLIPID (lipid injectable emulsion), for intravenous use Initial U.S. Approval: 2016
e0e9d917-ff6f-488e-a6a8-0ad59dd17d80
HUMAN PRESCRIPTION DRUG LABEL
Aug 15, 2025
Fresenius Kabi USA, LLC
DUNS: 608775388
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Smoflipid
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY – SMOFlipid**®**1000 mL Bag Label
SMOFlipid**®**
(lipid injectable emulsion, USP), 20%
200 grams/1000 mL (0.2 grams/mL)
Pharmacy Bulk Package.
Not for Direct Infusion
For intravenous use.
1000 mL
Energy: 2000 kcal per 1000 mL
Rx only
NDC 63323-820-05

INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
SMOFlipid is indicated in adult and pediatric patients, including term and preterm neonates, as a source of calories and essential fatty acids for parenteral nutrition (PN) when oral or enteral nutrition is not possible, insufficient, or contraindicated.
SMOFlipid is indicated in adult and pediatric patients, including term and preterm neonates, as a source of calories and essential fatty acids for parenteral nutrition when oral or enteral nutrition is not possible, insufficient, or contraindicated. (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
Use of SMOFlipid is contraindicated in patients with:
•
Known hypersensitivity to fish, egg, soybean, peanut or any of the active or inactive ingredients in SMOFlipid [see Warnings and Precautions (5.3)]
•
Severe disorders of lipid metabolism characterized by hypertriglyceridemia (serum triglyceride >1,000 mg/dL) [see Warnings and Precautions (5.7)]
•
Known hypersensitivity to fish, egg, soybean, peanut or any of the active or inactive ingredients. (4)
•
Severe disorders of lipid metabolism characterized by hypertriglyceridemia (serum triglycerides >1,000 mg/dL). (4, 5.7)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Clinical Decompensation with Rapid Infusion of Intravenous Lipid
Emulsion in Neonates and Infants
In the postmarketing setting, serious adverse reactions including acute respiratory distress, metabolic acidosis, and death have been reported in neonates and infants after rapid infusion of intravenous lipid emulsions. Hypertriglyceridemia was commonly reported.
Strictly adhere to the recommended total daily dosage; the hourly infusion rate should not exceed 0.75 mL/kg/hour [see Dosage and Administration (2.3)].
Preterm and small for gestational age infants have poor clearance of intravenous lipid emulsion and increased free fatty acid plasma levels following lipid emulsion infusion.
Carefully monitor the infant's ability to eliminate the infused lipids from the circulation (e.g., measure serum triglycerides and/or plasma free fatty acid levels). If signs of poor clearance of lipids from the circulation occur, stop the infusion and initiate a medical evaluation [see Warnings and Precautions (5.5, 5.7) and Overdosage (10)].
5.2 Parenteral Nutrition-Associated Liver Disease and Other Hepatobiliary
Disorders
Risk of Parenteral Nutrition-Associated Liver Disease
Parenteral nutrition-associated liver disease (PNALD), also referred to as intestinal failure- associated liver disease (IFALD), can present as cholestasis or hepatic steatosis, and may progress to steatohepatitis with fibrosis and cirrhosis (possibly leading to chronic hepatic failure). The etiology of PNALD is multifactorial; however, intravenously administered phytosterols (plant sterols) contained in plant-derived lipid emulsions, including SMOFlipid, have been associated with development of PNALD.
In a randomized study of neonates and infants expected to be treated with PN for at least 28 days, parenteral nutrition-associated cholestasis (PNAC), a precursor to PNALD, developed less frequently in SMOFlipid-treated patients than in 100% soybean oil lipid emulsion-treated patients [see Adverse Reactions (6.1), Use in Specific Populations (8.4)].
Monitor liver tests in patients treated with SMOFlipid and consider discontinuation or dosage reduction if abnormalities occur.
Other Hepatobiliary Disorders
Hepatobiliary disorders including cholecystitis and cholelithiasis have developed in some PN- treated patients without preexisting liver disease.
Monitor liver tests when administering SMOFlipid. Patients developing signs of hepatobiliary disorders should be assessed early to determine whether these conditions are related to SMOFlipid use.
5.3 Hypersensitivity Reactions
SMOFlipid contains soybean oil, fish oil, and egg phospholipids, which may cause hypersensitivity reactions. Cross reactions have been observed between soybean and peanut. In postmarketing experience, anaphylaxis has been reported following SMOFlipid administration [see Adverse Reactions (6.2)].
SMOFlipid is contraindicated in patients with known hypersensitivity to fish, egg, soybean, peanut or any of the active or inactive ingredients in SMOFlipid [see Contraindications (4)]. If a hypersensitivity reaction occurs, stop infusion of SMOFlipid immediately and initiate appropriate treatment and supportive measures.
5.4 Infections
Lipid emulsions, such as SMOFlipid, can support microbial growth and are an independent risk factor for the development of catheter-related bloodstream infections. To decrease the risk of infectious complications, ensure aseptic techniques are used for catheter placement, catheter maintenance, and preparation and administration of SMOFlipid.
Monitor for signs and symptoms of infection including fever and chills, as well as laboratory test results that might indicate infection (including leukocytosis and hyperglycemia). Perform frequent checks of the intravenous catheter insertion site for edema, redness, and discharge.
5.5 Fat Overload Syndrome
Fat overload syndrome is a rare condition that has been reported with intravenous lipid injectable emulsions and is characterized by a sudden deterioration in the patient's condition (e.g., fever, anemia, leukopenia, thrombocytopenia, coagulation disorders, hyperlipidemia, hepatomegaly, deteriorating liver function, and central nervous system manifestations such as coma). A reduced or limited ability to metabolize lipids, accompanied by prolonged plasma clearance (resulting in higher lipid levels), may result in this syndrome. Although fat overload syndrome has been most frequently observed when the recommended lipid dose or infusion rate was exceeded, cases have also been described when the lipid formulation was administered according to instructions.
If signs or symptoms of fat overload syndrome occur, stop SMOFlipid. The syndrome is usually reversible when the infusion of the lipid emulsion is stopped.
5.6 Refeeding Syndrome
Administering PN to severely malnourished patients may result in refeeding syndrome, which is characterized by the intracellular shift of potassium, phosphorus, and magnesium as patients become anabolic. Thiamine deficiency and fluid retention may also develop. To prevent these complications, closely monitor severely malnourished patients and slowly increase their nutrient intake.
5.7 Hypertriglyceridemia
The use of SMOFlipid is contraindicated in patients with hypertriglyceridemia with serum triglyceride concentrations >1,000 mg/dL.
Patients with conditions such as inherited lipid disorders, obesity, diabetes mellitus, or metabolic syndromes have a higher risk of developing hypertriglyceridemia with the use of SMOFlipid. In addition, patients with hypertriglyceridemia may have worsening of their hypertriglyceridemia with administration of SMOFlipid. Excessive dextrose administration may further increase such risk.
Evaluate patients' capacity to metabolize and eliminate the infused lipid emulsion by measuring serum triglycerides before the start of infusion (baseline value) and regularly throughout treatment. If triglyceride levels are above 400 mg/dL in adults, stop the SMOFlipid infusion and monitor serum triglyceride levels to avoid clinical consequences of hypertriglyceridemia such as pancreatitis. In pediatric patients with hypertriglyceridemia, lower triglyceride levels (i.e., below 400 mg/dL) may be associated with adverse reactions. Monitor serum triglyceride levels to avoid potential complications with hypertriglyceridemia such as pancreatitis, lipid pneumonitis, and neurologic changes, including kernicterus.
To minimize the risk of new or worsening of hypertriglyceridemia, assess high- risk patients for their overall energy intake including other sources of lipids and dextrose, as well as concomitant drugs that may affect lipid and dextrose metabolism.
5.8 Aluminum Toxicity
SMOFlipid contains no more than 25 mcg/L of aluminum. Prolonged PN administration in patients with renal impairment may result in aluminum reaching toxic levels. Preterm neonates are at greater risk because their kidneys are immature and they require large amounts of calcium and phosphate solutions that contain aluminum.
Patients with impaired kidney function, including preterm neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day can accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading in these patients may occur at even lower rates of administration.
5.9 Essential Fatty Acid Deficiency
Treatment-emergent cases of moderate or severe essential fatty acid deficiency (EFAD) (defined as the triene [Mead acid] to tetraene [arachidonic acid] ratio
0.2 and >0.4, respectively) were not observed in pediatric clinical trials of SMOFlipid up to 28 days [see Adverse Reactions (6.1)].
However, cases of EFAD have been reported in adult and pediatric patients in the postmarketing period with the use of SMOFlipid. The median time to onset was greater than 28 days among cases that reported time to onset.
Monitor patients for laboratory evidence (e.g., abnormal fatty acid levels) and clinical symptoms of EFAD (e.g., skin manifestations, poor growth). Laboratory testing using the triene to tetraene ratio may not be adequate to diagnose EFAD, and assessment of individual fatty acid levels may be needed. Ensure patients are receiving recommended dosages of SMOFlipid to prevent EFAD [see Dosage and Administration (2.3), Description (11)].
5.10 Monitoring/Laboratory Tests
Throughout treatment, monitor serum triglycerides [see Warnings and Precautions (5.7)], fluid and electrolyte status, blood glucose, liver and kidney function, coagulation parameters, and complete blood count including platelets.
The lipids contained in SMOFlipid may interfere with some laboratory blood tests (e.g., hemoglobin, lactate dehydrogenase, bilirubin, oxygen saturation) if blood is sampled before lipids have cleared from the bloodstream. Conduct these blood tests at least 6 hours after stopping the infusion.
SMOFlipid contains vitamin K that may counteract anticoagulant activity [see Drug Interactions (7)].
•
Clinical Decompensation with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Acute respiratory distress, metabolic acidosis, and death after rapid infusion of intravenous lipid emulsions have been reported. (5.1, 8.4)
•
Parenteral Nutrition-Associated Liver Disease: Increased risk in patients who receive parenteral nutrition for greater than 2 weeks, especially preterm neonates. Monitor liver tests; if abnormalities occur, consider discontinuation or dosage reduction. (5.2, 6.1, 8.4)
•
Hypersensitivity Reactions: Monitor for signs or symptoms. Discontinue infusion if reactions occur. (5.3)
•
Risk of Infections, Fat Overload Syndrome, Refeeding Syndrome, Hypertriglyceridemia, and Essential Fatty Acid Deficiency: Monitor for signs and symptoms; monitor laboratory parameters. (5.4, 5.5, 5.6, 5.7, 5.9)
•
Aluminum Toxicity: Increased risk in patients with renal impairment, including preterm neonates. (5.8, 8.4)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
Adverse reactions described elsewhere in this Prescribing Information are:
•
Clinical Decompensation with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants [see Warnings and Precautions (5.1)]
•
Parenteral Nutrition-Associated Liver Disease and Other Hepatobiliary Disorders [see Warnings and Precautions (5.2)]
•
Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
•
Infections [see Warnings and Precautions (5.4)]
•
Fat Overload Syndrome [see Warnings and Precautions (5.5)]
•
Refeeding Syndrome [see Warnings and Precautions (5.6)]
•
Hypertriglyceridemia [see Warnings and Precautions (5.7)]
•
Aluminum Toxicity [see Warnings and Precautions (5.8)]
•
Essential Fatty Acid Deficiency [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety database for SMOFlipid includes exposure in 399 patients (229 adults; 170 pediatric) in 9 clinical trials. Adult patients were exposed for 5 days to 4 weeks in 5 clinical trials. The pooled population exposed to SMOFlipid was adult patients up to 89 years old (20 to 89 years of age), 43% female and 99% Caucasian. The most frequently reported medical histories in the SMOFlipid group were surgical and medical procedures (84%), neoplasms (57%), gastrointestinal disorders (53%), vascular disorders (37%), and infections and infestations (20%). SMOFlipid was used as a component of PN which also included dextrose, amino acids, vitamins, and trace elements. Two of the 5 studies in adults were performed with SMOFlipid as a component of PN delivered in a 3- chamber bag.
Table 2: Adverse Reactions in >1% of Adult Patients Treated with SMOFlipid|
Adverse Reaction |
Number of Patients in SMOFlipid Group (N=229) |
Number of Patients in Soybean Oil lipid emulsion Comparator Group (N=230) |
|
Nausea |
20 (9%) |
26 (11%) |
|
Vomiting |
15 (7%) |
12 (5%) |
|
Hyperglycemia |
12 (5%) |
5 (2%) |
|
Flatulence |
10 (4%) |
4 (2%) |
|
Pyrexia |
9 (4%) |
11 (5%) |
|
Abdominal pain |
8 (4%) |
5 (2%) |
|
Blood triglycerides increased |
6 (3%) |
4 (2%) |
|
Hypertension |
6 (3%) |
9 (4%) |
|
Sepsis |
5 (2%) |
4 (2%) |
|
Dyspepsia |
5 (2%) |
1 (0%) |
|
Urinary tract infection |
4 (2%) |
3 (1%) |
|
Anemia |
4 (2%) |
2 (1%) |
|
Device related infection |
4 (2%) |
2 (1%) |
Less common adverse reactions occurring in ≤1% of adult patients who received SMOFlipid were dyspnea, leukocytosis, diarrhea, pneumonia, cholestasis, dysgeusia, increased blood alkaline phosphatase, increased gamma- glutamyltransferase, increased C-reactive protein, tachycardia, liver function test abnormalities, headache, pruritus, dizziness, rash and thrombophlebitis.
The 170 patients treated with SMOFlipid in four pediatric trials consisted of 149 patients <28 days of age, 13 patients 28 days to <2 years of age, and 8 patients 2 to 7 years of age; the duration of exposure was 7 to 84 days. Forty five percent of the pediatric patients were female, and 89% were Caucasian. Most pediatric patients were preterm neonates with feeding intolerance or other conditions requiring short-term (<29 days) PN.
Table 3: Adverse Reactions in >1% of Pediatric Patients Treated with SMOFlipid|
Adverse Reaction |
Number of Patients in SMOFlipid Group (N=170) |
Number of Patients in Soybean Oil lipid emulsion Comparator Group (N=163) |
|
Anemia |
30 (18%) |
33 (20%) |
|
Vomiting |
16 (9%) |
16 (10%) |
|
Gamma-glutamyltransferase increased |
10 (6%) |
12 (7%) |
|
Nosocomial infection |
10 (6%) |
6 (4%) |
|
Cholestasis |
7 (4%) |
10 (6%) |
|
Pyrexia |
7 (4%) |
7 (4%) |
|
C-reactive protein increased |
6 (4%) |
7 (4%) |
|
Hyperbilirubinemia |
5 (3%) |
7 (4%) |
|
Abdominal pain |
4 (2%) |
5 (3%) |
|
Bilirubin conjugated increased |
3 (2%) |
7 (4%) |
|
Diarrhea |
3 (2%) |
4 (3%) |
|
Tachycardia |
3 (2%) |
4 (3%) |
|
Thrombocytopenia |
3 (2%) |
4 (3%) |
|
Hyperglycemia |
3 (2%) |
2 (1%) |
|
Sepsis |
3 (2%) |
2 (1%) |
Less common adverse reactions occurring in ≤1% of pediatric patients who received SMOFlipid were decreased hematocrit, metabolic acidosis, increased blood triglycerides, infection, increased blood alkaline phosphatase, increased alanine aminotransferase, fluid overload, hypertension, hypertriglyceridemia, and rash.
The hepatic safety of SMOFlipid was evaluated in Pediatric Study 1, a randomized, active- controlled, double-blind, parallel-group, multi-center study that included 152 neonates and
9 patients ranging in age from 29 to 153 days who were expected to require PN for at least 28 days.
PNAC (defined as direct bilirubin >2mg/dl with a second confirmed elevation
2mg/dl at least 7 days later) occurred in 2.4% (2/83) of SMOFlipid-treated patients and 11.5% (9/78) in soybean oil lipid emulsion-treated patients. Most PNAC events occurred in patients who were treated for longer than 28 days.
The estimated cumulative incidence of PNAC is shown in the Kaplan-Meier cumulative incidence curve in Figure 1 [see Clinical Trials (14)].
Figure 1 Cumulative Incidence Curve of Time to Parenteral Nutrition- Associated Cholestasis (PNAC) with Standard Error Bars
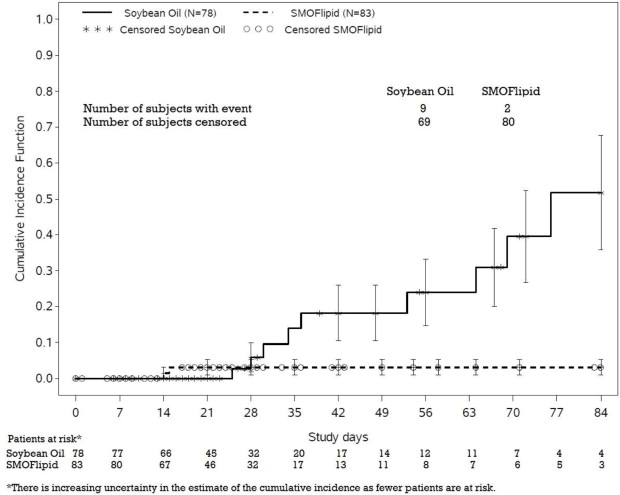
In the same trial, EFAD was determined by calculating the Holman index (the triene [Mead acid] to tetraene [arachidonic acid] ratio; T:T ratio). At the end of the first 28 days of treatment, 2.4% (2/83) SMOFlipid-treated patients and 2.6% (2/78) soybean oil lipid emulsion-treated patients had suspected EFAD (T:T ratio >0.05), and there were no cases of moderate (T:T ratio >0.2) or severe (T:T ratio >0.4) EFAD. There are insufficient data from this trial to determine the incidence of EFAD with duration of SMOFlipid treatment greater than 28 days because of substantial patient discontinuation from PN during the first 28 days.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of SMOFlipid in countries where it is registered. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to product exposure.
Cardiac disorders: palpitations
General disorders and administration site conditions: chills, chest pain, malaise
Hepatobiliary disorders: cholestasis
Infections and infestations: infection
Metabolism and nutrition disorders: fatty acid deficiency
Respiratory, thoracic and mediastinal disorders: dyspnea
Immune system disorders: hypersensitivity reactions, including anaphylaxis [see Contraindications (4), Warnings and Precautions (5.3)]
Skin and subcutaneous tissue disorders: hyperhidrosis
Vascular disorders: phlebitis
Most common adverse drug reactions (≥5%) from clinical trials in adults were nausea, vomiting, and hyperglycemia. Most common adverse drug reactions (≥5%) from clinical trials in pediatric patients were anemia, vomiting, increased gamma-glutamyltransferase, and nosocomial infection. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
Soybean and olive oils in SMOFlipid contain vitamin K1 which may counteract the anticoagulant activity of vitamin K antagonists such as warfarin. In patients who receive concomitant SMOFlipid and warfarin, increase monitoring of laboratory parameters for anticoagulant activity.
Vitamin K Antagonists (e.g., warfarin): Anticoagulant activity may be counteracted; increase monitoring of coagulation parameters. (7)
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
Warnings and Precautions (5.3) 7/2025
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Administration of the recommended dose of SMOFlipid is not expected to cause major birth defects, miscarriage, or other adverse maternal or fetal outcomes. No animal reproduction studies have been conducted with SMOFlipid. There are risks to the fetus associated with severe malnutrition during pregnancy (see Clinical Considerations).
The estimated background risk of major birth defects and miscarriage for the indicated population(s) is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo-Fetal Risk
Severe malnutrition in pregnant women is associated with preterm delivery, low birth weight, intrauterine growth restriction, congenital malformations, and perinatal mortality. Parenteral nutrition should be considered if the pregnant woman's nutritional requirements cannot be fulfilled by oral or enteral intake.
8.2 Lactation
Risk Summary
Administration of the recommended dose of SMOFlipid is not expected to cause harm to a breastfed infant. There are no data on the presence of SMOFlipid in human or animal milk or its effects on milk production. Available published literature includes fewer than five reported cases of breastfed infants exposed to various lipid emulsions via lactation, and these cases did not report adverse events. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for SMOFlipid and any potential adverse effects of SMOFlipid on the breastfed infant, or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of SMOFlipid have been established as a source of calories and essential fatty acids for PN when oral or enteral nutrition is not possible, insufficient, or contraindicated in pediatric patients, including term and preterm neonates. Use of SMOFlipid in neonates is supported by evidence from short-term (i.e., 1- to 4- week) studies, and one study following neonates beyond 4 weeks [see Clinical Studies (14.2)]. Use of SMOFlipid in older pediatric patients is supported by evidence from a short- term (i.e., <28 days) study in pediatric patients 28 days to 12 years of age and additional evidence from studies in adults [see Clinical Studies (14)]. The most common adverse reactions in SMOFlipid-treated pediatric patients were anemia, vomiting, gamma-glutamyltransferase increased, and nosocomial infection [see Adverse Reactions (6.1)].
PNALD, also referred to as IFALD, has been reported in pediatric patients who received SMOFlipid for more than 2 weeks. PNAC (a precursor to PNALD) was reported less frequently in SMOFlipid- treated patients compared to soybean oil lipid emulsion-treated patients in Pediatric Study 1 [see Warnings and Precautions (5.2) and Adverse Reactions (6.1)]. Although clinically significant cases of EFAD were not observed during short-term use in pediatric clinical studies, cases of EFAD have been reported with the use of SMOFlipid in the postmarketing setting [see Warnings and Precautions (5.9), Adverse Reactions (6.1)]. Monitor pediatric patients for laboratory evidence of EFAD because they may be particularly vulnerable to neurologic complications if adequate amounts of essential fatty acids are not provided [see Warnings and Precautions (5.9)].
In the postmarketing setting, clinical decompensation with rapid infusion of intravenous lipid emulsion in neonates and infants, sometimes fatal, has been reported [see Warnings and Precautions (5.1)]. Because of immature renal function, preterm infants receiving prolonged treatment with SMOFlipid may be at risk for aluminum toxicity [see Warnings and Precautions (5.8)].
8.5 Geriatric Use
Energy expenditure and requirements may be lower for older adults than younger patients. Of the 354 adult patients in clinical studies of SMOFlipid, 35% were
65 years of age and 10% were >75 years of age. No overall differences in the safety and efficacy of SMOFlipid were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity in some older patients cannot be ruled out.
OVERDOSAGE SECTION
10 OVERDOSAGE
In the event of an overdose, serious adverse reactions may result [see Warnings and Precautions (5.1, 5.5)]. Stop the infusion of SMOFlipid until triglyceride levels have normalized and symptoms have abated. The effects are usually reversible by stopping the lipid infusion. If medically appropriate, further intervention may be indicated. Lipids are not dialyzable from plasma.
DESCRIPTION SECTION
11 DESCRIPTION
SMOFlipid is a sterile, nonpyrogenic, white, homogenous lipid emulsion for intravenous infusion. The lipid content of SMOFlipid is 0.2 g/mL, and comprises a mixture of soybean oil, MCTs, olive oil, and fish oil. The mean concentration of linoleic acid (an omega-6 essential fatty acid) is 35 mg/mL (range of 28 to 50 mg/mL), and alpha-linolenic acid (an omega-3 essential fatty acid) is 4.5 mg/mL (range of 3 to 7 mg/mL). The phosphate content is 15 mmol/L.
The total energy content, including fat, phospholipids, and glycerol is 2,000 kcal/L.
Each 100 mL of SMOFlipid contains approximately 6 g soybean oil, 6 g MCT, 5 g olive oil, 3 g fish oil, 1.2 g egg phospholipids, 2.5 g glycerin, 16.3 to 22.5 mg all-rac-alpha-tocopherol, 0.03 g sodium oleate, water for injection, and sodium hydroxide for pH adjustment (pH 6 to 9).
SMOFlipid has an osmolality of approximately 380 mOsm/kg water (which represents an osmolarity of 270 mOsm/L).
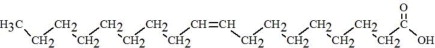
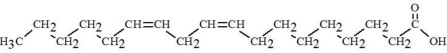
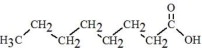
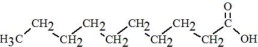
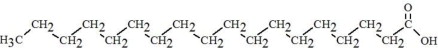
The oils included in SMOFlipid consist of a mixture of triglycerides of predominantly unsaturated fatty acids with the following structure:
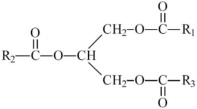
where 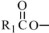 ,
,
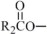 , and
, and 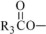 are saturated and
unsaturated fatty acid residues. The major components of the fatty acids in
SMOFlipid are oleic acid (23% to 35%), linoleic acid (14% to 25%), caprylic
acid (13% to 24%), palmitic acid (7% to 12%), capric acid (5% to 15%), stearic
acid (1.5% to 4%), alpha-linolenic acid (1.5% to 3.5%), eicosapentaenoic acid
(EPA; 1% to 3.5%), and docosahexaenoic acid (DHA; 1% to 3.5%).
are saturated and
unsaturated fatty acid residues. The major components of the fatty acids in
SMOFlipid are oleic acid (23% to 35%), linoleic acid (14% to 25%), caprylic
acid (13% to 24%), palmitic acid (7% to 12%), capric acid (5% to 15%), stearic
acid (1.5% to 4%), alpha-linolenic acid (1.5% to 3.5%), eicosapentaenoic acid
(EPA; 1% to 3.5%), and docosahexaenoic acid (DHA; 1% to 3.5%).
|
Oleic Acid C18H34O****2 |
|
|
Linoleic Acid C18H32O****2 |
|
|
Caprylic Acid C8H16O****2 |
|
|
Capric Acid C10H20O****2 |
|
|
Stearic Acid C18H36O****2 |
|
|
Palmitic Acid C16H32O****2 |
|
|
Linolenic Acid C18H30O****2 |
|
|
EPA C20H30O****2 |
|
|
DHA C22H32O****2 |
|
SMOFlipid contains no more than 25 mcg/L of aluminum.
The container is not made with natural rubber latex, PVC, or DEHP.
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
•
SMOFlipid is prepared and administered by a healthcare provider in the inpatient setting. Patients and caregivers may prepare and administer SMOFlipid for home use after appropriate training by a trained healthcare provider.
•
SMOFlipid is for intravenous infusion into a central or peripheral vein.
•
Do not exceed the recommended maximum infusion rate in Table 1[see Dosage and Administration (2.3) and Warnings and Precautions (5.1)].
•
SMOFlipid admixtures with osmolarity
ͦ
Greater than or equal to 900 mOsm/L must be infused through a central vein.
ͦ
Less than 900 mOsm/L may be administered either through a central or peripheral vein.
•
Use a 1.2 micron in-line filter during administration.
•
Use a dedicated infusion line without any connections. Do not connect multiple medications in series.
•
To prevent air embolism, use a nonvented infusion set or close the vent on a vented set and fully evacuate residual gas in the bag prior to administration.
•
Do not pressurize the flexible bag to increase flow rates, and if administration is controlled by a pumping device, turn off the pump before the bag runs dry.
•
Do not use infusion sets and lines that contain di-2-ethylhexyl phthalate (DEHP), including infusion sets that contain polyvinyl chloride (PVC) components, because they contain DEHP as a plasticizer.
•
SMOFlipid can be infused concurrently into the same vein as dextrose-amino acid solutions (as part of PN) by a Y-connector located near the infusion site; flow rates of each solution should be controlled separately by infusion pumps.
•
After connecting the infusion set, start infusion of SMOFlipid immediately. Complete the infusion within 12 hours when using a Y-connector and within 24 hours when used as part of an admixture.
2.2 Preparation Instructions
Use the following instructions to prepare single-dose SMOFlipid 100 mL, 250 mL, and 500 mL Flexible Containers for administration:
|
|
• • 2. • • • • 3. • • • • • • • 4. • • |
SMOFlipid 100 mL, 250 mL and 500 mL single-dose Flexible Containers
•
After removing the overpouch, infuse immediately. If not used immediately, the product should be stored at 2°C to 8°C (36°F to 46°F) for no longer than 24 hours. After removal from storage, infuse within 12 hours when using a Y-connector and within 24 hours when used as part of an admixture.
SMOFlipid 1000 mL Pharmacy Bulk Package
•
For admixing use only and not for direct intravenous infusion. Prior to administration, transfer to a separate PN container for individual patient use.
•
Transfer the contents through the blue infusion port using a suitable sterile transfer device or dispensing set. Discard any unused contents.
•
Use the Pharmacy Bulk Package immediately for admixing after removal from overpouch. If not used immediately, the product can be stored for no longer than 24 hours at 2°C to 8°C (36°F to 46°F). After removal from storage, and once the closure is penetrated, use Pharmacy Bulk Package contents within 4 hours.
Admixing Instructions
•
Prepare the admixture in PN containers using strict aseptic techniques to avoid microbial contamination.
•
Do not add SMOFlipid to the PN container first; destabilization of the lipid may occur. The prime destabilizers of emulsions are excessive acidity (such as a pH <5) and inappropriate electrolyte content. Amino acid solutions exert buffering effects that protect the emulsion from destabilization. Give careful consideration to the addition of divalent cations (Ca++ and Mg++), which have been shown to cause emulsion instability.
•
Do not inject additives directly into SMOFlipid.
•
SMOFlipid may be mixed with amino acid and dextrose injections to produce “all-in-one” PN admixtures. The mixing sequence below must be followed for manual compounding to minimize pH-related problems by ensuring that typically acidic dextrose injections are not mixed with lipid emulsions alone; shake bags gently after each addition.
ͦ
Transfer dextrose injection to the PN container.
ͦ
Transfer amino acid injection.
ͦ
Transfer SMOFlipid.
•
Simultaneous transfer of amino acid injection, dextrose injection, and SMOFlipid to the PN container is also permitted; follow automated compounding device instructions as indicated. Use gentle agitation during admixing to minimize localized concentration effects.
•
Additions to the PN admixtures should be evaluated by a pharmacist for compatibility. Questions about compatibility may be directed to Fresenius Kabi USA, LLC.
•
Inspect the admixture to ensure that precipitates have not formed during preparation of the admixture and the emulsion has not separated. Discard the admixture if any of the above are observed.
•
Infuse admixtures containing SMOFlipid immediately. If not used immediately, store admixtures under refrigeration at 2°C to 8°C (36°F to 46°F) for no longer than 24 hours. Infusion must be complete within 24 hours after removal from refrigeration. Discard any remaining admixture.
•
Protect the admixed PN solution from light.
2.3 Recommended Dosage and Administration
•
The recommended nutritional requirements of lipid and recommended dosages of SMOFlipid to be administered to meet those requirements for pediatric and adult patients are provided in Table 1, along with recommendations for the initial and maximum infusion rates.
•
The recommended duration of infusion for SMOFlipid will vary depending on the clinical situation. Adjust the administration flow rate by taking into account the dose being administered, the daily volume/intake, and the duration of the infusion [see Overdosage (10)].
•
When determining dose, energy supplied by dextrose and amino acids from PN, as well as energy from oral or enteral nutrition, has to be taken into account. Energy and lipid provided from lipid-based medications should also be taken into account (e.g., propofol).
•
Prior to administration of SMOFlipid, correct severe fluid and electrolyte disorders and measure serum triglyceride levels to establish a baseline value. In patients with elevated triglyceride levels, initiate SMOFlipid at a lower dosage and titrate in smaller increments, monitoring the triglyceride levels with each adjustment [see Warnings and Precautions (5.7)].
•
SMOFlipid contains 0.162 to 0.225 mg/mL of all-rac-alpha-tocopherol. Take into account the amount of all-rac-alpha-tocopherol in SMOFlipid when determining the need for additional supplementation.
|
*The neonatal period is defined as including term, post-term, and preterm newborn infants. The neonatal period for term and post-term infants is the day of birth plus 27 days. For preterm infants, the neonatal period is defined as the day of birth through the expected age of delivery plus 27 days (i.e., 44 weeks post-menstrual age). | |||
|
** Daily dosage should also not exceed a maximum of 60% of total energy requirements [see Overdosage (10)]. | |||
|
Age |
Nutritional Requirements |
Direct Infusion Rate | |
|
Recommended Initial Dosage and Maximum Dosage |
Initial |
Maximum | |
|
Birth to 2 years of age (including preterm and term neonates*) |
Initial 0.5 to 1 g/kg/day not to exceed 3 g/kg/day** |
0.1 to 0.2 mL/kg/hour for the first 15 to 30 minutes; gradually increase to the required rate after 30 minutes |
0.75 mL/kg/hour |
|
Pediatric patients 2 to <12 years of age |
Initial 1 to 2 g/kg/day |
0.2 to 0.4 mL/kg/hour for the first 15 to 30 minutes; gradually increase to the required rate after 30 minutes |
0.75 mL/kg/hour |
|
Pediatric patients 12 to 17 years of age |
Initial 1 g/kg/day |
0.2 to 0.4 mL/kg/hour for the first 15 to 30 minutes; gradually increase to the required rate after 30 minutes |
0.75 mL/kg/hour |
|
Adults |
1 to 2 g/kg/day |
0.2 mL/kg/hour for the first 15 to 30 minutes; gradually increase to the required rate after 30 minutes |
0.5 mL/kg/hour |
•
For intravenous infusion into a central or peripheral vein. (2.1)
•
SMOFlipid Pharmacy Bulk Package is only indicated for use in pharmacy admixture programs for the preparation of three-in-one or total nutrition admixtures. (2.2)
•
Protect the admixed PN solution from light. (2.2)
•
Recommended dosage depends on age, energy expenditure, clinical status, body weight, tolerance, ability to metabolize and eliminate lipids, and consideration of additional energy given to the patient. (2.3)
•
For information on the age-appropriate infusion rate, see the full prescribing information (2.3, 5.1)
|
Age |
Nutritional Requirements | |
|
Initial Recommended Dosage |
Maximum Dosage | |
|
Birth to 2 years of age (including preterm and term neonates) |
0.5 to 1 g/kg/day |
3 g/kg/day |
|
Pediatric patients 2 to <12 years of age |
1 to 2 g/kg/day |
3 g/kg/day |
|
Pediatric patients 12 to 17 years of age |
1 g/kg/day |
2.5 g/kg/day |
|
Adults |
1 to 2 g/kg/day |
2.5 g/kg/day |
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
SMOFlipid is a sterile, homogenous lipid injectable emulsion in Flexible Containers supplied as:
•
20 g of lipid/100 mL in 100 mL single-dose Flexible Container
•
50 g of lipid/250 mL in 250 mL single-dose Flexible Container
•
100 g of lipid/500 mL in 500 mL single-dose Flexible Container
•
200 g of lipid/1000 mL in 1000 mL Pharmacy Bulk Package
SMOFlipid is a sterile, homogenous lipid injectable emulsion supplied as 20 g of lipid/100 mL in 100 mL single-dose Flexible Container, 50 g of lipid/250 mL in 250 mL single-dose Flexible Container, 100 g of lipid/500 mL in 500 mL single-dose Flexible Container, and 200 g of lipid/1000 mL in 1000 mL Pharmacy Bulk Package. (3)
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
SMOFlipid provides a biologically utilizable source of calories and essential fatty acids.
Fatty acids serve as an important substrate for energy production. The most common mechanism of action for energy production derived from fatty acid metabolism is beta oxidation. Fatty acids are also important for membrane structure and function, as precursors for bioactive molecules (such as prostaglandins), and as regulators of gene expression.
12.2 Pharmacodynamics
The pharmacodynamic effects of SMOFlipid have not been fully characterized.
12.3 Pharmacokinetics
SMOFlipid provides fatty acids in the form of triglycerides which are hydrolyzed by lipoprotein lipase to release free fatty acids. Alpha-linolenic acid and linoleic acid are metabolized within a common biochemical pathway through a series of desaturation and elongation steps. Downstream products of alpha-linolenic acid are EPA and DHA, and linoleic acid is converted to arachidonic acid.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies with SMOFlipid have not been performed to evaluate the carcinogenic potential or effects on fertility.
No mutagenic effects were observed in the following in vitro studies with SMOFlipid: bacterial gene mutation assay in Salmonella typhimurium, chromosomal aberration assay in human lymphocytes, and hypoxanthine phosphoribosyl transferase (HPRT) gene mutation assay in V79 cells.
In an in vivo bone marrow cytogenic study in rats, no mutagenic effect was observed.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Adult Clinical Studies
The efficacy of SMOFlipid compared to soybean oil lipid emulsions was evaluated in 3 clinical studies in adult patients. Nutritional efficacy in adult studies was assessed by changes in anthropometric indices (body weight, height, and body mass index [BMI]), changes in lipid and protein metabolism (albumin), and fatty acid parameters. Of the 354 adult patients (176 SMOFlipid; 178 comparator), 62% were male, 99% were Caucasian, and ages ranged from 19 to 96 years. All patients received SMOFlipid or the comparator as part of a PN regimen. Although Adult Study 1, Adult Study 2, and Adult Study 3 were not adequately designed to demonstrate the noninferiority of SMOFlipid to the soybean oil comparator, they support SMOFlipid as a source of calories and essential fatty acids in adults. The lipid dosage was variable in these studies and adjusted to the patient's nutritional requirements.
Adult Study 1 was a double-blind, randomized, active-controlled, parallel- group, multicenter study in patients who required PN for at least 28 days. Seventy-five patients were enrolled, and 73 patients were treated with either SMOFlipid or a soybean oil lipid emulsion. Changes in mean triglyceride levels from baseline values to Week 4 were similar in both the SMOFlipid and comparator groups. Mean albumin levels demonstrated a comparable decrease in both groups. Mean changes in body weight (kg) and BMI (kg/m2) were similar in both the SMOFlipid and comparator group.
Adult Study 2 was a phase 3, randomized, double-blind, active-controlled, multicenter study. A total of 249 postoperative adult patients were randomized to receive either SMOFlipid or a soybean oil intravenous lipid emulsion for at least 5 days as part of their total parenteral nutrition (TPN) regimen. From baseline to Day 6, mean triglyceride levels increased similarly in both the SMOFlipid and comparator groups.
Adult Study 3 was a double-blind randomized, active-controlled, parallel- group, single-center study in 32 adult patients who required TPN for 10 to 14 days. Patients were treated with either SMOFlipid or a soybean oil lipid emulsion. The increase in mean triglyceride levels from baseline to the final assessment was similar in both the SMOFlipid and comparator groups.
14.2 Pediatric Clinical Studies
The efficacy of SMOFlipid compared to soybean oil lipid emulsions in pediatric patients of all age groups, including term and preterm neonates, was evaluated in 333 pediatric patients in 4 randomized active-controlled, double-blind, parallel-group controlled clinical studies. Although Pediatric Studies 1, 2, 3, and 4 were not adequately designed to demonstrate the noninferiority of SMOFlipid to the soybean oil comparator, they support SMOFlipid as a source of calories and essential fatty acids in pediatric patients. The 333 pediatric patients (170 SMOFlipid; 163 comparator) consisted of 296 patients who were <28 days old, 22 patients 29 days to <2 years old, and 15 patients 2 to <12 years old. Fifty percent of the pediatric patients were male and 87% were Caucasian. All patients received SMOFlipid or the comparator as part of a PN regimen. Nutritional efficacy in neonates was assessed by changes in anthropometric indices (body weight, height, head circumference). Nutritional efficacy in pediatric patients, 28 days to 12 years of age, was assessed by changes in triglyceride concentrations and fatty acid parameters.
Pediatric Study 1 enrolled 152 preterm and term neonates (birth up to 28 days) and 9 patients ranging in age from 29 to 153 days. Patients were treated with either SMOFlipid (n=83) or a soybean oil lipid emulsion (n=78). A total of 119 patients (61 SMOFlipid; 58 comparator) received study treatment for ≥14 days. A total of 25 patients received SMOFlipid for ≥29 days; 5 patients received SMOFlipid for the maximum study duration of 78-84 days.
Pediatric Studies 2 and 3 enrolled 60 and 84 preterm neonates, respectively, who were treated with either SMOFlipid or a soybean oil lipid emulsion (72 neonates in each group). The median treatment duration for SMOFlipid group was 12 days in Pediatric Study 2 and 7 days in Pediatric Study 3.
Pediatric Study 4 enrolled 13 patients 5 months to <2 years of age and 15 patients 2 to 11.5 years of age. Patients were treated with either SMOFlipid (n=15) or a soybean oil lipid emulsion (n=13) with a median treatment duration of 27 days.
In Pediatric Studies 1, 2 and 3, which enrolled neonates, SMOFlipid-treated patients showed increases in the median body weight, height/length, and head circumference (which was measured in Studies 1 and 3) comparable to the soybean oil lipid emulsion-treated patients. Mean triglyceride levels from baseline to the final assessment in Pediatric Studies 1, 2, and 3 were variable in these neonates, but overall differences between groups were not considered clinically relevant. Mean triglyceride levels in Pediatric Study 4 were variable, but remained within the normal range.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
SMOFlipid is a sterile lipid injectable emulsion with a lipid content of 0.2 gram/mL available in Flexible Containers in the following sizes:
|
Product Code |
Unit of Sale |
Strength |
Each |
|
830307310 |
NDC 63323-820-00 |
20 grams/100 mL |
NDC 63323-820-01 |
|
830307312 |
NDC 63323-820-12 |
20 grams/100 mL |
NDC 63323-820-02 |
|
830570310 |
NDC 63323-820-74 |
50 grams/250 mL |
NDC 63323-820-04 |
|
830820310 |
NDC 63323-820-50 |
100 grams/500 mL |
NDC 63323-820-03 |
|
830920310 |
NDC 63323-820-10 |
200 grams/1000 mL |
NDC 63323-820-05 |
Store below 25°C (77°F). Avoid excessive heat. Do not freeze. If accidentally frozen, discard container. Store in overpouch until ready for use.
SMOFlipid 100 mL, 250 mL and 500 mL single-dose Flexible Containers.
After removing the overpouch, infuse immediately. If not used immediately, the product should be stored at 2°C to 8°C (36°F to 46°F) for no longer than 24 hours. After removal from storage, infuse within 12 hours when using a Y-connector or within 24 hours if used as part of an admixture [see Dosage and Administration (2.2)].
SMOFlipid 1000 mL Pharmacy Bulk Package
Use the Pharmacy Bulk Package immediately for admixing after removal from overpouch. If not used immediately, the product should be stored for no longer than 24 hours at 2°C to 8°C (36°F to 46°F). After removal from storage, and once the closure is penetrated, use Pharmacy Bulk Package contents within 4 hours [see Dosage and Administration (2.2)].
Admixtures
Infuse admixtures containing SMOFlipid immediately. If not used immediately, admixtures should be stored at 2°C to 8°C (36°F to 46°F) for no longer than 24 hours. After removal from storage, infuse within 24 hours [see Dosage and Administration (2.2)].
Protect the admixed PN solution from light [see Dosage and Administration (2.2)].
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
When initiating SMOFlipid administration, discuss the following information:
Clinical Decompensation with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants
Inform caregivers that acute respiratory distress and death may occur in neonates and infants after rapid infusion of intravenous lipid emulsions. If SMOFlipid is infused at home, instruct caregivers not to exceed the maximum infusion rate [see Warnings and Precautions (5.1)].
Parenteral Nutrition-Associated Liver Disease and Other Hepatobiliary Disorders
Inform patients and caregivers that use of parenteral nutrition may result in parenteral nutrition- associated liver disease and/or other hepatobiliary disorders [see Warnings and Precautions (5.2)].
Hypersensitivity Reactions
Inform patients and caregivers that SMOFlipid may cause hypersensitivity reactions, including anaphylaxis. If SMOFlipid is infused at home, instruct patients or caregivers to stop the infusion of SMOFlipid immediately and seek medical attention if they experience signs or symptoms of a hypersensitivity reaction, such as rapid or weak heartbeat, feeling faint, difficulty in breathing or swallowing, vomiting, nausea, headache, sweating, dizziness, hives, rash, itching, flushing, dizziness, fever, or chills [see Warnings and Precautions (5.3)].
Infections
Inform patients and caregivers that patients who receive SMOFlipid are at risk of infection. If SMOFlipid is infused at home, instruct patients or caregivers to ensure aseptic techniques are used for the preparation and administration of SMOFlipid and to monitor for signs and symptoms of infection [see Warnings and Precautions (5.4)].
Fat Overload Syndrome
Inform patients and caregivers that fat overload syndrome has been reported with the use of intravenous lipid emulsions. If SMOFlipid is infused at home, instruct patients or caregivers to stop SMOFlipid if signs or symptoms of fat overload syndrome occur [see Warnings and Precautions (5.5)].
Refeeding Syndrome
If the patient is severely malnourished, inform patients and caregivers that administering parenteral nutrition including SMOFlipid may result in refeeding syndrome. [see Warnings and Precautions (5.6)].
Hypertriglyceridemia
Inform patients and caregivers about the risks of hypertriglyceridemia with SMOFlipid use [see Warnings and Precautions (5.7)].
Aluminum Toxicity
Inform patients and caregivers that prolonged PN administration in patients with renal impairment, including preterm neonates, may result in aluminum reaching toxic levels associated with central nervous system and bone toxicity [see Warnings and Precautions (5.8)].
Essential Fatty Acid Deficiency
Inform patients and caregivers that cases of EFAD with the use of SMOFlipid have been reported in adults and pediatric patients in the postmarketing period [see Warnings and Precautions (5.9)].
Preparation and Administration Instructions
If it is acceptable for a patient or caregiver to administer SMOFlipid at home, then provide recommendations on how to prepare, add compatible additives (when appropriate), administer, and store SMOFlipid [see Dosage and Administration (2.1, 2.2)].
Manufactured by:

Uppsala, Sweden
Fresenius Kabi, Oxalert, and SMOFlipid are registered trademarks of Fresenius
Kabi, www.fresenius-kabi.com/us
451418F