EXTRA STRENGTH SIMETHICONE
c1b88a30-ffeb-431c-ab73-728cdc786de0
HUMAN OTC DRUG LABEL
Sep 1, 2025
Bi-Mart
DUNS: 027630078
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Simethicone
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel
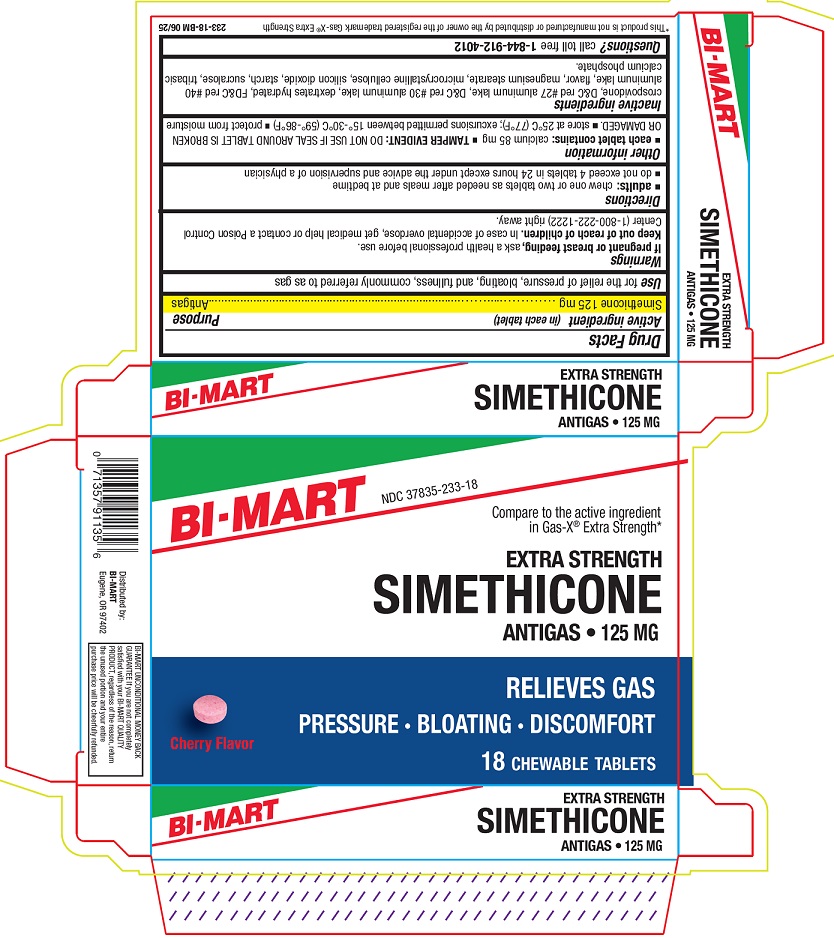
INDICATIONS & USAGE SECTION
Uses
for the relief of pressure, bloating, and fullness commonly referred to as gas
OTC - ACTIVE INGREDIENT SECTION
Active ingredient (in each tablet)
Simethicone 125 mg
OTC - PURPOSE SECTION
Purpose
Antigas
WARNINGS SECTION
Warnings
OTC - PREGNANCY OR BREAST FEEDING SECTION
If pregnant or breast feeding,
ask a health professional before use.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children.
In case of accidental overdose, get medical help or a Poison Control Center (1-800-222-1222) right away.
DOSAGE & ADMINISTRATION SECTION
Directions
***adults:**chew one or two tablets as needed after meals and at bedtime
- do not exceed 4 tablets in 24 hours except under the advice and supervision of a physician
STORAGE AND HANDLING SECTION
Other information
***each tablet contains:**calcium 85 mg ***TAMPER EVIDENT:**DO NOT USE IF SEAL AROUN TABLET IS BROKEN OR DAMAGED
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- protect from moisture
INACTIVE INGREDIENT SECTION
Inactive ingredients
crospovidone, D&C red #27 aluminum lake, D&C red #30 aluminum lake, dextrates hydrated, FD&C red #40 aluminum lake, flavor, magnesium stearate, microcrystalline cellulose, silicon dioxide, starch, sucralose, tribasic calcium phosphate.
OTC - QUESTIONS SECTION
Questions?
call toll free1-844-912-4012
