Argentum nitricum
Argentum nitricum 5C
8460da09-6080-130f-e053-2991aa0afaf4
HUMAN OTC DRUG LABEL
Sep 23, 2025
Boiron
DUNS: 282560473
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
SILVER NITRATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
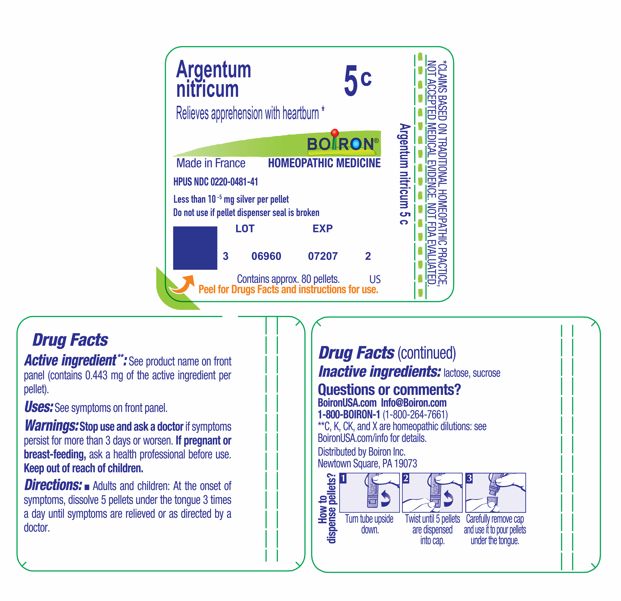


INDICATIONS & USAGE SECTION
Relieves apprehension with heartburn *
OTC - ACTIVE INGREDIENT SECTION
Argentum nitricum 5C HPUS
Less than 10 -5 mg silver per pellet
Active ingredient**: See product name on front panel (contains 0.443 mg of the active ingredient per pellet).
OTC - PURPOSE SECTION
WARNINGS SECTION
OTC - STOP USE SECTION
Stop use and ask a doctor ifsymptoms persist for more than 3 days or worsen.
OTC - PREGNANCY OR BREAST FEEDING SECTION
If pregnant or breast-feeding, ask a health professional before use.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children.
DOSAGE & ADMINISTRATION SECTION
Adults and children: At the onset of symptoms, dissolve 5 pellets under the tongue 3 times a day until symptoms are relieved or as directed by a doctor.
INACTIVE INGREDIENT SECTION
lactose, sucrose
OTC - QUESTIONS SECTION
1-800-BOIRON-1 (1-800-264-7661),
BoironUSA.com Info@boiron.com
Distributed by Boiron, Inc. Newtown Square, PA 19073
SPL UNCLASSIFIED SECTION
Do not use if pellet dispenser seal is broken.
Contains approx 80 pellets.
How to dispense pellets? Turn tube upside down. Twist until 5 pellets are
dispensed into cap. Carefully remove the cap and use it to pour pellets under
the tongue.
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
*C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details.
