NewVite
NEWVITE
87b08d72-b7a1-4a97-a47d-fe2770cb7364
DIETARY SUPPLEMENT
Jun 23, 2025
Trivia Pharmaceuticals, LLC
DUNS: 119353862
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Vitamin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
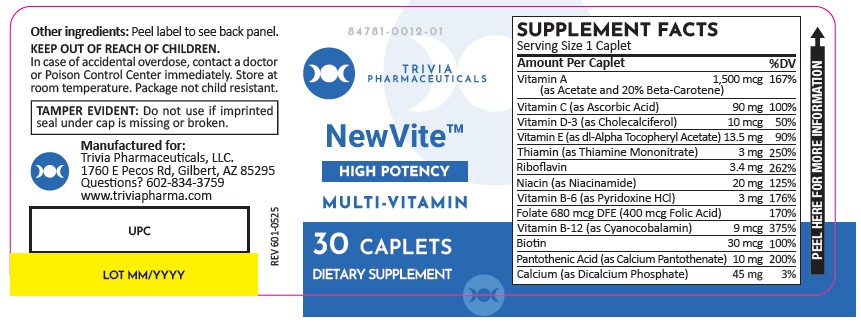
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Label:
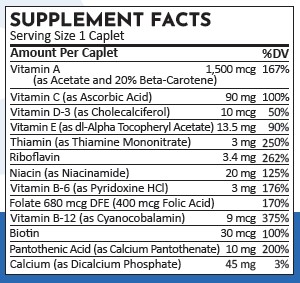
HEALTH CLAIM SECTION
Health Claim Section:
NewVite Tablets
Other ingredients:
microcrystalline cellulose, gelatin, stearic acid, hypromellose,
croscarmellose sodium, starch, silicon dioxide, magnesium stearate, dicalcium
phosphate, FD&C Red #40 lake, sucrose, mineral oil, polyethylene glycol,
titanium dioxide, sodium ascorbate, FD&C Yellow #6 lake, tocopherols,
triglycerides, tricalcium phosphate, sodium benzoate, BHT, ascorbyl palmitate,
sorbic acid, and talc.
STATEMENT OF IDENTITY SECTION
Description:
NewVite Tablets are supplied as brown, oblong tablets dispensed in plastic bottles of 30 ct.
84781-012-01
Reserved for Professional Recommendation
All prescriptions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product. This product may be administered only under a physician’s supervision. There are no implied or explicit claims on therapeutic equivalence.
Manufactured for:
Trivia Pharmaceuticals
Gilbert, AZ
WARNINGS SECTION
Warnings and Precautions:
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
As with any supplement, if you are pregnant, nursing, taking medication, or have a medical condition, ask a health professional before taking this product.
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
DOSAGE & ADMINISTRATION SECTION
Dosage and Administration:
Adults - One caplet daily as a dietary supplement, preferably with a meal, or as recommended by your health professional.
PRECAUTIONS SECTION
Precautions:
CONTRAINDICATIONS
This product is contraindicated in patients with known hypersensitivity to any
of the ingredients.
PRECAUTIONS
This product is contraindicated in patients with a known hypersensitivity to
any of the ingredients.
ADVERSE REACTIONS
Allergic sensitizations have been reported following oral administration of
folic acid. Consult your physician immediately if adverse side effects occur.
KEEP OUT OF THE REACH OF CHILDREN.
SAFE HANDLING WARNING SECTION
Storage:
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F).
