Pure velocity MINERAL SUNSCREEN SPF 50
4003047b-bc55-bb11-e063-6294a90aa433
HUMAN OTC DRUG LABEL
Sep 30, 2025
Guangzhou Haishi Biological Technology Co., Ltd.
DUNS: 421262738
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
TITANIUM DIOXIDE,ZINC OXIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (31)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
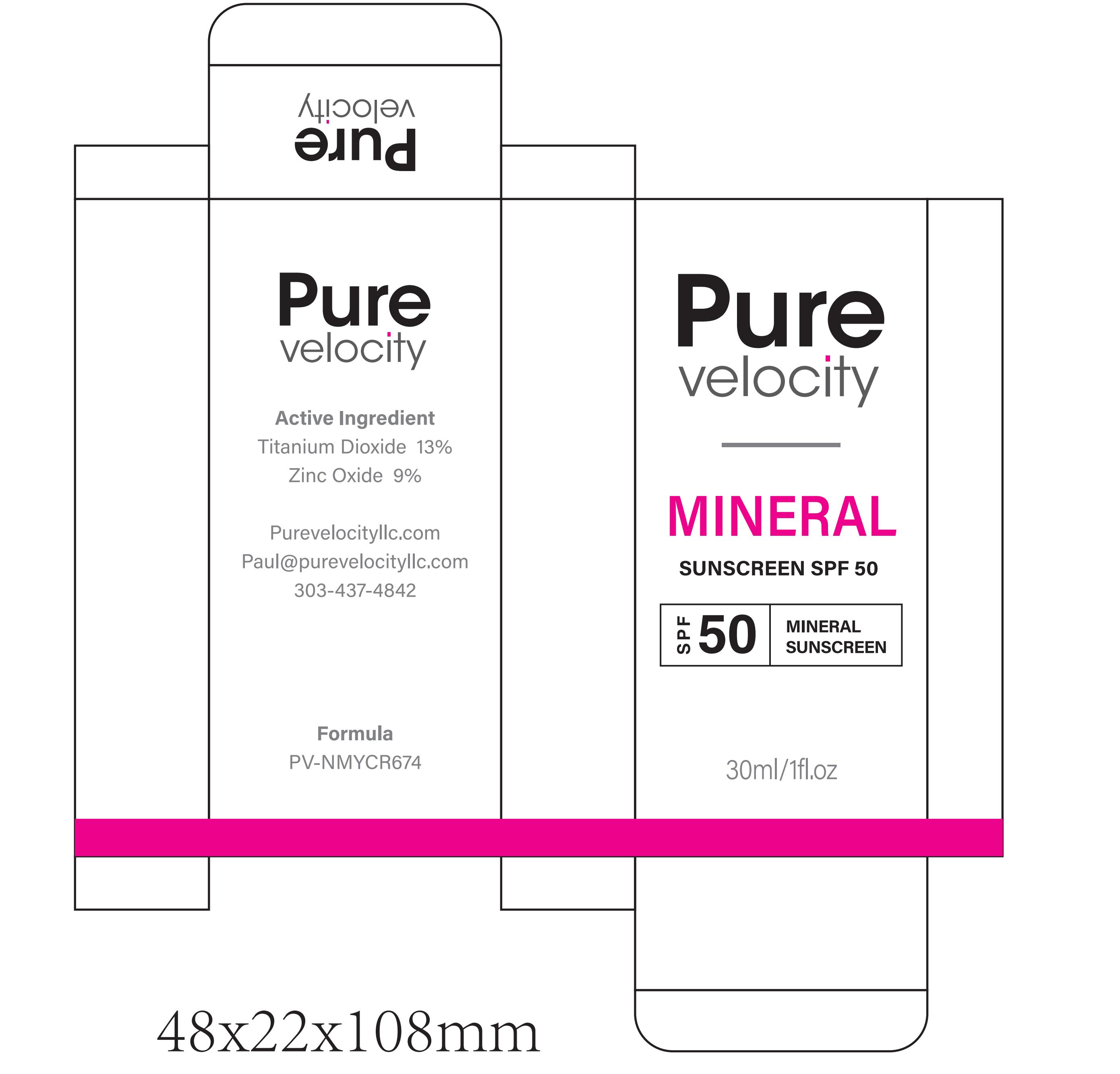
INDICATIONS & USAGE SECTION
Uses
If used as directed with other sun protection measures(see Directions),
decreases the risk of skin cancer and early skin aging caused by the sun.
OTC - ACTIVE INGREDIENT SECTION
Active Ingredients
TITANIUM DIOXIDE
ZINC OXIDE
OTC - PURPOSE SECTION
Purpose
SUNSCREEN
WARNINGS SECTION
Warnings
For external use only
OTC - STOP USE SECTION
Stop Use
Rinse with wate to remove.
Stop use and ask doctor if rash occurs.
OTC - DO NOT USE SECTION
Do Not Use
Do not use on damaged or broken skin.
OTC - WHEN USING SECTION
When Using
When using this product keep out of eyes.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep Out Of Reach Of Children
Keep Out Of Reach Of Children
INACTIVE INGREDIENT SECTION
Inactive ingredients
WATER
ASCORBIC ACID
TITANIUM DIOXIDE
ZINC OXIDE
NIACINAMIDE
PROPYLENE GLYCOL
GLYCERIN
TREHALOSE
ALLANTOIN
SODIUM HYALURONATE
DIPOTASSIUM GLYCYRRHIZATE
LIMNANTHES ALBA (MEADOWFOAM) SEED OIL
MACADAMIA TERNIFOLIA SEED OIL
TRITICUM VULGARE (WHEAT) GERM OIL
ETHYLHEXYL SALICYLATE
C12-15 ALKYL BENZOATE
CETEARYL ALCOHOL
CETEARYL GLUCOSIDE
CETEARETH-21
DIMETHICONE
TOCOPHERYL ACETATE
BISABOLOL
XANTHAN GUM
ETHYLHEXYLGLYCERIN
CHLORPHENESIN
HYDROLYZED BETA-GLUCAN
PAEONIA ALBIFLORA ROOT EXTRACT
TRITICUM VULGARE (WHEAT) GERM EXTRACT
CENTELLA ASIATICA ROOT EXTRACT
ALOE FEROX LEAF EXTRACT
PARFUM
DOSAGE & ADMINISTRATION SECTION
Dosage & administration
Squeeze out an appropriate amount of Sunscreen and spread evenly on skin.
