Xalatan
These highlights do not include all the information needed to use XALATAN safely and effectively. See full prescribing information for XALATAN. XALATAN (latanoprost ophthalmic solution) 0.005%, for topical ophthalmic useInitial U.S. Approval: 1996
f4e73059-5ba0-4d73-9ea1-09d8d654e844
HUMAN PRESCRIPTION DRUG LABEL
Dec 27, 2022
PFIZER LABORATORIES DIV PFIZER INC
DUNS: 134489525
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
latanoprost
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
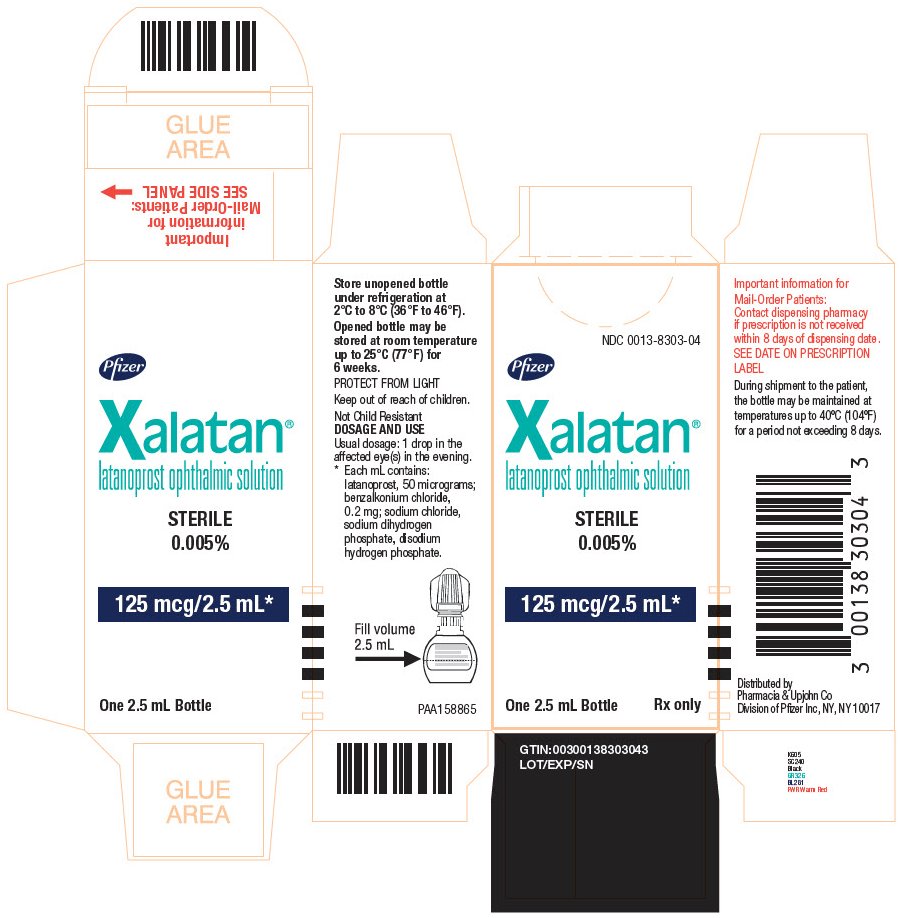
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 2.5 mL
NDC 0013-8303-04
Pfizer
Xalatan**®**
latanoprost ophthalmic solution
STERILE
0.005%
125 mcg/2.5 mL*
One 2.5 mL Bottle Rx only
Store unopened bottle
under refrigeration at
2°C to 8°C (36°F to 45°F).
Opened bottle may be
stored at room temperature
up to 25°C (77°F) for
6 weeks.
PROTECT FROM LIGHT
Keep out of reach of children.
Not Child Resistant
DOSAGE AND USE
Usual doeage: 1 drop in the
affected eye(s) in the evening.
- Each mL contains:
latanoprost, 50 micorgrams:
benzalkonium chloride,
0.2 mg; sodium chloride,
sodium dihydrogen
phosphate, disodium
hydrogen phosphate.
Important information for
Mail-Order Patients:
Contact dispensing pharmacy
if prescription is not received
within 8 days of dispensing date.
SEE DATE ON PRESCRIPTION
LABEL
During shipment to the patient,
the bottle may be maintained at
temperatures up to 40°C (104°F)
for a period not exceeding 8 days.
Distributed by
Pharmacia & Upjohn Co
Division of Pfizer Inc, NY, NY 10017
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Elevated Baseline IOP
Patients with mean baseline IOP of 24 – 25 mmHg who were treated for 6 months in multi-center, randomized, controlled trials demonstrated 6 – 8 mmHg reductions in IOP. This IOP reduction with XALATAN 0.005% dosed once daily was equivalent to the effect of timolol 0.5% dosed twice daily.
14.2 Progression of Increased Iris Pigmentation
A 3-year open-label, prospective safety study with a 2-year extension phase was conducted to evaluate the progression of increased iris pigmentation with continuous use of XALATAN once-daily as adjunctive therapy in 519 patients with open-angle glaucoma. The analysis was based on observed-cases population of the 380 patients who continued in the extension phase.
Results showed that the onset of noticeable increased iris pigmentation occurred within the first year of treatment for the majority of the patients who developed noticeable increased iris pigmentation. Patients continued to show signs of increasing iris pigmentation throughout the 5 years of the study. Observation of increased iris pigmentation did not affect the incidence, nature, or severity of adverse events (other than increased iris pigmentation) recorded in the study. IOP reduction was similar regardless of the development of increased iris pigmentation during the study.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Potential for Pigmentation
Advise patients about the potential for increased brown pigmentation of the iris, which may be permanent. Inform patients about the possibility of eyelid skin darkening, which may be reversible after discontinuation of XALATAN [see Warnings and Precautions (5.1)].
Potential for Eyelash Changes
Inform patients of the possibility of eyelash and vellus hair changes in the treated eye during treatment with XALATAN. These changes may result in a disparity between eyes in length, thickness, pigmentation, number of eyelashes or vellus hairs, and/or direction of eyelash growth. Eyelash changes are usually reversible upon discontinuation of treatment.
Handling the Container
Instruct patients to avoid allowing the tip of the dispensing container to contact the eye or surrounding structures because this could cause the tip to become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions [see Warnings and Precautions (5.6)].
When to Seek Physician Advice
Advise patients that if they develop an intercurrent ocular condition (e.g., trauma or infection) or have ocular surgery, or develop any ocular reactions, particularly conjunctivitis and eyelid reactions, they should immediately seek their physician’s advice concerning the continued use of the multiple-dose container.
Contact Lens Use
Advise patients that XALATAN contains benzalkonium chloride, which may be absorbed by contact lenses. Contact lenses should be removed prior to administration of the solution. Lenses may be reinserted 15 minutes following administration of XALATAN.
Use with Other Ophthalmic Drugs
Advise patients that if more than one topical ophthalmic drug is being used, the drugs should be administered at least five (5) minutes apart.
If a Dose is Missed
Advise patients that if one dose is missed, treatment should continue with the next dose as normal.
This product’s labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
Pfizer Manufacturing Belgium NV
Puurs, Belgium
LAB-0135-14.1
