Donepezil
These highlights do not include all the information needed to use donepezil hydrochloride tablets safely and effectively. See full prescribing information for donepezil hydrochloride tablets. DONEPEZIL hydrochloride tablets USP, for oral use Initial U.S. Approval: 1996
51c9bd8b-d603-54a0-e054-00144ff8d46c
HUMAN PRESCRIPTION DRUG LABEL
Jan 14, 2022
NuCare Pharmaceuticals, Inc.
DUNS: 010632300
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Donepezil hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL 10 mg

INDICATIONS & USAGE SECTION
1 INDICATIONS & USAGE
Donepezil hydrochloride tablet, USP is indicated for the treatment of dementia of the Alzheimer's type. Efficacy has been demonstrated in patients with mild, moderate, and severe Alzheimer's disease.
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
Donepezil hydrochloride tablet is contraindicated in patients with known hypersensitivity to donepezil hydrochloride or to piperidine derivatives.
Known hypersensitivity to donepezil hydrochloride or to piperidine derivatives ( 4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Anesthesia
Donepezil hydrochloride, as a cholinesterase inhibitor, is likely to exaggerate succinylcholine-type muscle relaxation during anesthesia.
5.2 Cardiovascular Conditions
Because of their pharmacological action, cholinesterase inhibitors may have vagotonic effects on the sinoatrial and atrioventricular nodes. This effect may manifest as bradycardia or heart block in patients both with and without known underlying cardiac conduction abnormalities. Syncopal episodes have been reported in association with the use of donepezil hydrochloride.
5.3 Nausea and Vomiting
Donepezil hydrochloride, as a predictable consequence of its pharmacological properties, has been shown to produce diarrhea, nausea, and vomiting. These effects, when they occur, appear more frequently with the 10 mg/day dose than with the 5 mg/day dose.
Although in most cases, these effects have been transient, sometimes lasting one to three weeks, and have resolved during continued use of donepezil hydrochloride, patients should be observed closely at the initiation of treatment and after dose increases.
5.4 Peptic Ulcer Disease and GI Bleeding
Through their primary action, cholinesterase inhibitors may be expected to increase gastric acid secretion due to increased cholinergic activity. Therefore, patients should be monitored closely for symptoms of active or occult gastrointestinal bleeding, especially those at increased risk for developing ulcers, e.g., those with a history of ulcer disease or those receiving concurrent nonsteroidal anti-inflammatory drugs (NSAIDs). Clinical studies of donepezil hydrochloride in a dose of 5 mg/day to 10 mg/day have shown no increase, relative to placebo, in the incidence of either peptic ulcer disease or gastrointestinal bleeding.
5.6 Genitourinary Conditions
Although not observed in clinical trials of donepezil hydrochloride, cholinomimetics may cause bladder outflow obstruction.
5.7 Neurological Conditions: Seizures
Cholinomimetics are believed to have some potential to cause generalized convulsions. However, seizure activity also may be a manifestation of Alzheimer's disease.
5.8 Pulmonary Conditions
Because of their cholinomimetic actions, cholinesterase inhibitors should be prescribed with care to patients with a history of asthma or obstructive pulmonary disease.
•Cholinesterase inhibitors are likely to exaggerate succinylcholine-type
muscle relaxation during anesthesia ( 5.1).
•Cholinesterase inhibitors may have vagotonic effects on the sinoatrial and
atrioventricular nodes manifesting as bradycardia or heart block ( 5.2).
•Donepezil hydrochloride can cause vomiting. Patients should be observed
closely at initiation of treatment and after dose increases ( 5.3).
•Patients should be monitored closely for symptoms of active or occult
gastrointestinal (GI) bleeding, especially those at increased risk for
developing ulcers ( 5.4).
•Cholinomimetics may cause bladder outflow obstructions ( 5.6).
•Cholinomimetics are believed to have some potential to cause generalized
convulsions ( 5.7).
•Cholinesterase inhibitors should be prescribed with care to patients with a
history of asthma or obstructive pulmonary disease ( 5.8).
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in
the labeling:
• Cardiovascular Conditions [see Warnings and Precautions ( 5.2)]
• Nausea and Vomiting [see Warnings and Precautions ( 5.3)]
• Peptic Ulcer Disease and GI Bleeding [see Warnings and Precautions ( 5.4)]
• Genitourinary Conditions [see Warnings and Precautions ( 5.6)]
• Neurological Conditions: Seizures [see Warnings and Precautions ( 5.7)]
• Pulmonary Conditions [see Warnings and Precautions ( 5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse
reaction rates observed in the clinical trials of a drug cannot be directly
compared to rates in the clinical trials of another drug and may not reflect
the rates observed in practice.
Donepezil hydrochloride has been administered to over 1,700 individuals during
clinical trials worldwide. Approximately 1200 of these patients have been
treated for at least 3 months and more than 1,000 patients have been treated
for at least 6 months. Controlled and uncontrolled trials in the United States
included approximately 900 patients. In regards to the highest dose of 10
mg/day, this population includes 650 patients treated for 3 months, 475
patients treated for 6 months, and 116 patients treated for over 1 year. The
range of patient exposure is from 1 to 1,214 days
Mild to Moderate Alzheimer's Disease
Adverse Reactions Leading to Discontinuation
The rates of discontinuation from controlled clinical trials of donepezil
hydrochloride due to adverse reactions for the donepezil hydrochloride 5
mg/day treatment groups were comparable to those of placebo treatment groups
at approximately 5%. The rate of discontinuation of patients who received
7-day escalations from 5 mg/day to 10 mg/day was higher at 13%.
The most common adverse reactions leading to discontinuation, defined as those
occurring in at least 2% of patients and at twice or more the incidence seen
in placebo patients, are shown in Table 1.
|
Table 1. Most Common Adverse Reactions Leading to Discontinuation in Patients with Mild to Moderate Alzheimer's Disease | |||
|
Adverse Reaction |
Placebo (n=355) % |
5 mg/day |
10 mg/day |
|
Nausea |
1 |
1 |
3 |
|
Diarrhea |
0 |
<1 |
3 |
|
Vomiting |
<1 |
<1 |
2 |
Most Common Adverse Reactions
The most common adverse reactions, defined as those occurring at a frequency
of at least 5% in patients receiving 10 mg/day and twice the placebo rate, are
largely predicted by donepezil hydrochloride's cholinomimetic effects. These
include nausea, diarrhea, insomnia, vomiting, muscle cramp, fatigue and
anorexia. These adverse reactions were often transient, resolving during
continued donepezil hydrochloride treatment without the need for dose
modification.
There is evidence to suggest that the frequency of these common adverse
reactions may be affected by the rate of titration. An open-label study was
conducted with 269 patients who received placebo in the 15- and 30-week
studies. These patients were titrated to a dose of 10 mg/day over a 6-week
period. The rates of common adverse reactions were lower than those seen in
patients titrated to 10 mg/day over one week in the controlled clinical trials
and were comparable to those seen in patients on 5 mg/day.
See Table 2 for a comparison of the most common adverse reactions following
one and six week titration regimens.
|
Table 2. Comparison of Rates of Adverse Reactions in Mild to Moderate Patients Titrated to 10 mg/day over 1 and 6 Weeks | ||||
|
No titration |
One week titration |
Six week titration | ||
|
Adverse Event |
Placebo (n=315) |
5 mg/day (n=311) |
10 mg/day (n=315) |
10 mg/day (n=269) |
|
Nausea |
6 |
5 |
19 |
6 |
|
Diarrhea |
5 |
8 |
15 |
9 |
|
Insomnia |
6 |
6 |
14 |
6 |
|
Fatigue |
3 |
4 |
8 |
3 |
|
Vomiting |
3 |
3 |
8 |
5 |
|
Muscle cramps |
2 |
6 |
8 |
3 |
|
Anorexia |
2 |
3 |
7 |
3 |
Table 3 lists adverse reactions that occurred in at least 2% of patients in pooled placebo-controlled trials who received either donepezil hydrochloride 5 mg or 10 mg and for which the rate of occurrence was greater for patients treated with donepezil hydrochloride than with placebo. In general, adverse reactions occurred more frequently in female patients and with advancing age.
|
Table 3. Adverse Reactions in Pooled Placebo-Controlled Clinical Trials in Mild to Moderate Alzheimer’s Disease | ||
|
Adverse Reaction |
Placebo |
Donepezil Hydrochloride (n=747) |
|
Percent of Patients with any Adverse Reaction |
72 |
74 |
|
Nausea |
6 |
11 |
|
Diarrhea |
5 |
10 |
|
Headache |
9 |
10 |
|
Insomnia |
6 |
9 |
|
Pain, various locations |
8 |
9 |
|
Dizziness |
6 |
8 |
|
Accident |
6 |
7 |
|
Muscle Cramps |
2 |
6 |
|
Fatigue |
3 |
5 |
|
Vomiting |
3 |
5 |
|
Anorexia |
2 |
4 |
|
Ecchymosis |
3 |
4 |
|
Abnormal Dreams |
0 |
3 |
|
Depression |
<1 |
3 |
|
Weight loss |
1 |
3 |
|
Arthritis |
1 |
2 |
|
Frequent Urination |
1 |
2 |
|
Somnolence |
<1 |
2 |
|
Syncope |
1 |
2 |
Severe Alzheimer's Disease (Donepezil Hydrochloride 5 mg/day and 10 mg/day)
Donepezil hydrochloride has been administered to over 600 patients with severe
Alzheimer’s disease during clinical trials of at least 6 months duration,
including three double-blind, placebo-controlled trials, two of which had an
open label extension.
Adverse Reactions Leading to Discontinuation
The rates of discontinuation from controlled clinical trials of donepezil
hydrochloride due to adverse reactions for the donepezil hydrochloride
patients were approximately 12% compared to 7% for placebo patients. The most
common adverse reactions leading to discontinuation, defined as those
occurring in at least 2% of donepezil hydrochloride patients and at twice or
more the incidence seen in placebo, were anorexia (2% vs. 1% placebo), nausea
(2% vs. <1% placebo), diarrhea (2% vs. 0% placebo) and urinary tract infection
(2% vs. 1% placebo).
Most Common Adverse Reactions
The most common adverse reactions, defined as those occurring at a frequency
of at least 5% in patients receiving donepezil hydrochloride and at twice or
more the placebo rate, are largely predicted by donepezil hydrochloride’s
cholinomimetic effects. These include diarrhea, anorexia, vomiting, nausea,
and ecchymosis. These adverse reactions were often transient, resolving during
continued donepezil hydrochloride treatment without the need for dose
modification.
Table 4 lists adverse reactions that occured in at least 2% of patients in
pooled placebo-controlled trials who received donepezil hydrochloride 5 mg or
10 mg and for which the rate of occurrence was greater for patients treated
with donepezil hydrochloride than with placebo.
|
Table 4. Adverse Reactions in Pooled Controlled Clinical Trials in Severe Alzheimer’s Disease | ||
|
Body System/Adverse Reactions |
Placebo (n=392) % |
** Donepezil Hydrochloride (n=501)** % |
|
Percent of Patients with any Adverse Reactions |
73 |
81 |
|
Accident |
12 |
13 |
|
Infection |
9 |
11 |
|
Diarrhea |
4 |
10 |
|
Anorexia |
4 |
8 |
|
Vomiting |
4 |
8 |
|
Nausea |
2 |
6 |
|
Insomnia |
4 |
5 |
|
Ecchymosis |
2 |
5 |
|
Headache |
3 |
4 |
|
Hypertension |
2 |
3 |
|
Pain |
2 |
3 |
|
Back Pain |
2 |
3 |
|
Eczema |
2 |
3 |
|
Hallucinations |
1 |
3 |
|
Hostility |
2 |
3 |
|
Increase in Creatine Phosphokinase |
1 |
3 |
|
Nervousness |
2 |
3 |
|
Fever |
1 |
2 |
|
Chest Pain |
<1 |
2 |
|
Confusion |
1 |
2 |
|
Dehydration |
1 |
2 |
|
Depression |
1 |
2 |
|
Dizziness |
1 |
2 |
|
Emotional Lability |
1 |
2 |
|
Hemorrhage |
1 |
2 |
|
Hyperlipemia |
<1 |
2 |
|
Personality Disorder |
1 |
2 |
|
Somnolence |
1 |
2 |
|
Syncope |
1 |
2 |
|
Urinary Incontinence |
1 |
2 |
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use
donepezil hydrochloride. Because these reactions are reported voluntarily from
a population of uncertain size, it is not always possible to reliably estimate
their frequency or establish a causal relationship to drug exposure.
Abdominal pain, agitation, aggression, cholecystitis, confusion, convulsions,
hallucinations, heart block (all types), hemolytic anemia, hepatitis,
hyponatremia, neuroleptic malignant syndrome, pancreatitis, rash,
rhabdomyolysis, QTc prolongation, and torsade de pointes.
Most common adverse reactions in clinical studies of donepezil hydrochloride are nausea, diarrhea, insomnia, vomiting, muscle cramps, fatigue, and anorexia ( 6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Hetero Labs Limited at 866-495-1995 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Use with Anticholinergics
Because of their mechanism of action, cholinesterase inhibitors have the potential to interfere with the activity of anticholinergic medications.
7.2 Use with Cholinomimetics and Other Cholinesterase Inhibitors
A synergistic effect may be expected when cholinesterase inhibitors are given concurrently with succinylcholine, similar neuromuscular blocking agents or cholinergic agonists such as bethanechol.
•Cholinesterase inhibitors have the potential to interfere with the activity
of anticholinergic medications ( 7.1).
•A synergistic effect may be expected with concomitant administration of
succinylcholine, similar neuromuscular blocking agents, or cholinergic
agonists ( 7.2).
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE & ADMINISTRATION
2.1 Dosing in Mild to Moderate Alzheimer's Disease
The recommended starting dosage of donepezil hydrochloride tablet is 5 mg administered once per day in the evening, just prior to retiring. The maximum recommended dosage of donepezil hydrochloride tablet in patients with mild to moderate Alzheimer's disease is 10 mg per day. A dose of 10 mg should not be administered until patients have been on a daily dose of 5 mg for 4 to 6 weeks.
2.2 Dosing in Severe Alzheimer's Disease
The recommended starting dosage of donepezil hydrochloride tablet is 5 mg administered once per day in the evening, just prior to retiring. A dose of 10 mg should not be administered until patients have been on a daily dose of 5 mg for 4 to 6 weeks.
2.3 Administration Information
Donepezil hydrochloride tablet should be taken in the evening, just prior to retiring. Donepezil hydrochloride tablet can be taken with or without food.
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS & STRENGTHS
Donepezil hydrochloride is supplied as film coated, round tablets containing 5
mg, or 10 mg of donepezil hydrochloride.
The 5 mg tablets are white round biconvex, film coated tablets debossed with
"I" on one side and "24" on the other side.
The 10 mg tablets are yellow round biconvex, film coated tablets debossed with
"I" on one side and "21" on the other side.
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
There are no adequate or well-controlled studies in pregnant women. Donepezil
hydrochloride should be used during pregnancy only if the potential benefit
justifies the potential risk to the fetus.
Oral administration of donepezil to pregnant rats and rabbits during the
period of organogenesis did not produce any teratogenic effects at doses up to
10 mg/kg/day (approximately 7 times the MRHD on a mg/m 2 basis). Oral
administration of donepezil (1, 3, 10 mg/kg/day) to rats during late gestation
and throughout lactation to weaning produced an increase in stillbirths and
reduced offspring survival through postpartum day 4 at the highest dose. The
no-effect dose of 3 mg/kg/day is approximately equal to the MRHD on a mg/m 2
basis.
8.3 Nursing Mothers
It is not known whether donepezil is excreted in human milk. Caution should be exercised when donepezil hydrochloride is administered to a nursing woman.
8.4 Pediatric Use
The safety and effectiveness of donepezil hydrochloride in pediatric patients have not been established.
8.5 Geriatric Use
Alzheimer's disease is a disorder occurring primarily in individuals over 55 years of age. The mean age of patients enrolled in the clinical studies with donepezil hydrochloride was 73 years; 80% of these patients were between 65 and 84 years old, and 49% of patients were at or above the age of 75. The efficacy and safety data presented in the clinical trials section were obtained from these patients. There were no clinically significant differences in most adverse reactions reported by patient groups ≥ 65 years old and < 65 years old.
Pregnancy: Based on animal data, donepezil hydrochloride may cause fetal harm
( 8.1).
See 17 for PATIENT COUNSELING INFORMATION and FDA- approved patient labelling.
OVERDOSAGE SECTION
10 OVERDOSAGE
Because strategies for the management of overdose are continually evolving, it is advisable to contact a Poison Control Center to determine the latest recommendations for the management of an overdose of any drug.
As in any case of overdose, general supportive measures should be utilized.
Overdosage with cholinesterase inhibitors can result in cholinergic crisis
characterized by severe nausea, vomiting, salivation, sweating, bradycardia,
hypotension, respiratory depression, collapse and convulsions. Increasing
muscle weakness is a possibility and may result in death if respiratory
muscles are involved. Tertiary anticholinergics such as atropine may be used
as an antidote for donepezil hydrochloride overdosage. Intravenous atropine
sulfate titrated to effect is recommended: an initial dose of 1 to 2 mg IV
with subsequent doses based upon clinical response. A typical responses in
blood pressure and heart rate have been reported with other cholinomimetics
when co-administered with quaternary anticholinergics such as glycopyrrolate.
It is not known whether donepezil hydrochloride and/or its metabolites can be
removed by dialysis (hemodialysis, peritoneal dialysis, or hemofiltration).
Dose-related signs of toxicity in animals included reduced spontaneous
movement, prone position, staggering gait, lacrimation, clonic convulsions,
depressed respiration, salivation, miosis, tremors, fasciculation and lower
body surface temperature.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No evidence of carcinogenic potential was obtained in an 88-week
carcinogenicity study of donepezil conducted in mice at oral doses up to 180
mg/kg/day (approximately 40 times the maximum recommended human dose on a mg/m
2 basis), or in a 104-week carcinogenicity study in rats at oral doses up to
30 mg/kg/day (approximately 13 times the MRHD on a mg/m 2 basis).
Donepezil was negative in a battery of genotoxicity assays ( in vitro
bacterial reverse mutation, in vitro mouse lymphoma tk, in vitro chromosomal
aberration, and in vivo mouse micronucleus).
Donepezil had no effect on fertility in rats at oral doses up to 10 mg/kg/day
(approximately 4 times the MRHD on a mg/m 2 basis) when administered to males
and females prior to and during mating and continuing in females through
implantation.
13.2 Animal Toxicology and/ or Pharmacology
In an acute dose neurotoxicity study in female rats, oral administration of
donepezil and memantine in combination resulted in increased incidence,
severity, and distribution of neurodegeneration compared with memantine alone.
The no-effect levels of the combination were associated with clinically
relevant plasma donepezil and memantine levels.
The relevance of this finding to humans is unknown.
DESCRIPTION SECTION
11 DESCRIPTION
Donepezil hydrochloride USP is a reversible inhibitor of the enzyme acetylcholinesterase, known chemically as 2, 3-Dihydro-5, 6-dimethoxy-2-[[1-(phenylmethyl)-4-piperidinyl]methyl]-1H-inden-1-one hydrochloride. Donepezil hydrochloride USP is commonly referred to in the pharmacological literature as E2020. It has an empirical formula of C 24H 29NO 3HCl and a molecular weight of 415.96. Donepezil hydrochloride USP is a white to off white crystalline powder and is soluble in water, methanol and chloroform and sparingly soluble in acetic acid.
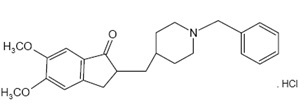
Donepezil hydrochloride USP is available for oral administration in film-
coated tablets containing 5 and 10 mg of donepezil hydrochloride USP.
Inactive ingredients in 5 mg and 10 mg tablets are corn starch, hydroxypropyl
cellulose, lactose monohydrate, magnesium stearate and microcrystalline
cellulose. The film coating contains hypromellose, polyethylene glycol, talc
and titanium dioxide. Additionally, the 10 mg tablet contains yellow iron
oxide as a coloring agent.
Meets Organic Impurities Test Procedure 2
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Current theories on the pathogenesis of the cognitive signs and symptoms of
Alzheimer's disease attribute some of them to a deficiency of cholinergic
neurotransmission.
Donepezil hydrochloride is postulated to exert its therapeutic effect by
enhancing cholinergic function. This is accomplished by increasing the
concentration of acetylcholine through reversible inhibition of its hydrolysis
by acetylcholinesterase. There is no evidence that donepezil alters the course
of the underlying dementing process.
12.3 Pharmacokinetics
Pharmacokinetics of donepezil are linear over a dose range of 1 to 10 mg given
once daily. The rate and extent of absorption of donepezil hydrochloride
tablets are not influenced by food.
Based on population pharmacokinetic analysis of plasma donepezil
concentrations measured in patients with Alzheimer's disease, following oral
dosing, peak plasma concentration is achieved in 3 hours for donepezil
hydrochloride 10 mg tablets.
The elimination half life of donepezil is about 70 hours, and the mean
apparent plasma clearance (Cl/F) is 0.13 to 0.19 L/hr/kg. Following multiple
dose administration, donepezil accumulates in plasma by 4 to 7 fold, and
steady state is reached within 15 days. The steady state volume of
distribution is 12 to 16 L/kg. Donepezil is approximately 96% bound to human
plasma proteins, mainly to albumins (about 75%) and alpha1 - acid glycoprotein
(about 21%) over the concentration range of 2 to 1000 ng/mL.
Donepezil is both excreted in the urine intact and extensively metabolized to
four major metabolites, two of which are known to be active, and a number of
minor metabolites, not all of which have been identified. Donepezil is
metabolized by CYP 450 isoenzymes 2D6 and 3A4 and undergoes glucuronidation.
Following administration of 14C-labeled donepezil, plasma radioactivity,
expressed as a percent of the administered dose, was present primarily as
intact donepezil (53%) and as 6-O-desmethyl donepezil (11%), which has been
reported to inhibit AChE to the same extent as donepezil in vitro and was
found in plasma at concentrations equal to about 20% of donepezil.
Approximately 57% and 15% of the total radioactivity was recovered in urine
and feces, respectively, over a period of 10 days, while 28% remained
unrecovered, with about 17% of the donepezil dose recovered in the urine as
unchanged drug. Examination of the effect of CYP2D6 genotype in Alzheimer's
patients showed differences in clearance values among CYP2D6 genotype
subgroups. When compared to the extensive metabolizers, poor metabolizers had
a 31.5% slower clearance and ultra-rapid metabolizers had a 24% faster
clearance.
Hepatic Disease
In a study of 10 patients with stable alcoholic cirrhosis, the clearance of
donepezil hydrochloride was decreased by 20% relative to 10 healthy age-and
sex-matched subjects.
Renal Disease
In a study of 11 patients with moderate to severe renal impairment (ClC < 18
mL/min/1.73 m 2) the clearance of donepezil hydrochloride did not differ from
11 age- and sex-matched healthy subjects.
Age
No formal pharmacokinetic study was conducted to examine age-related
differences in the pharmacokinetics of donepezil hydrochloride. Population
pharmacokinetic analysis suggested that the clearance of donepezil in patients
decreases with increasing age. When compared with 65-year old, subjects,
90-year old subjects have a 17% decrease in clearance, while 40-year old
subjects have a 33% increase in clearance. The effect of age on donepezil
clearance may not be clinically significant.
Gender and Race
No specific pharmacokinetic study was conducted to investigate the effects of
gender and race on the disposition of donepezil hydrochloride. However,
retrospective pharmacokinetic analysis and population pharmacokinetic analysis
of plasma donepezil concentrations measured in patients with Alzheimer's
disease indicates that gender and race (Japanese and Caucasians) did not
affect the clearance of donepezil hydrochloride to an important degree.
Body weight
There was a relationship noted between body weight and clearance. Over the
range of body weight from 50 kg to 110 kg, clearance increased from 7.77 L/h
to 14.04 L/h, with a value of 10 L/hr for 70 kg individuals.
Drug Interactions
Effect of donepezil hydrochloride on the Metabolism of Other Drugs
No in vivo clinical trials have investigated the effect of donepezil
hydrochloride on the clearance of drugs metabolized by CYP 3A4 (e.g.
cisapride, terfenadine) or by CYP 2D6 (e.g. imipramine). However, in vitro
studies show a low rate of binding to these enzymes (mean K i about 50 to130
µM), that, given the therapeutic plasma concentrations of donepezil (164 nM),
indicates little likelihood of interference. Based on in vitro studies,
donepezil shows little or no evidence of direct inhibition of CYP2B6, CYP2C8
and CYP2C19 at clinically relevant concentrations.
Whether donepezil hydrochloride has any potential for enzyme induction is not
known. Formal pharmacokinetic studies evaluated the potential of donepezil
hydrochloride for interaction with theophylline, cimetidine, warfarin, digoxin
and ketoconazole. No effects of donepezil hydrochloride on the
pharmacokinetics of these drugs were observed.
Effect of Other Drugs on the Metabolism of Donepezil Hydrochloride
Ketoconazole and quinidine, strong inhibitors of CYP450 3A and 2D6,
respectively, inhibit donepezil metabolism in vitro. Whether there is a
clinical effect of quinidine is not known. Population pharmacokinetic analysis
showed that in the presence of concomitant CYP2D6 inhibitors donepezil AUC was
increased by approximately 17% to 20% in Alzheimer's disease patients taking
donepezil hydrochloride 10 mg. This represented an average effect of weak,
moderate, and strong CYP2D6 inhibitors. In a 7-day crossover study in 18
healthy volunteers, ketoconazole (200 mg q.d.) increased mean donepezil (5 mg
q.d.) concentrations (AUC 0 to 24 and C max) by 36%. The clinical relevance of
this increase in concentration is unknown.
Inducers of CYP 3A (e.g., phenytoin, carbamazepine, dexamethasone, rifampin,
and phenobarbital) could increase the rate of elimination of donepezil
hydrochloride.
Formal pharmacokinetic studies demonstrated that the metabolism of donepezil
hydrochloride is not significantly affected by concurrent administration of
digoxin or cimetidine.
An in vitro study showed that donepezil was not a substrate of P-glycoprotein.
Drugs Highly Bound to Plasma Proteins
Drug displacement studies have been performed in vitro between this highly
bound drug (96%) and other drugs such as furosemide, digoxin, and warfarin.
donepezil hydrochloride at concentrations of 0.3-10 micrograms/mL did not
affect the binding of furosemide (5 micrograms/mL), digoxin (2 ng/mL), and
warfarin (3 micrograms/mL) to human albumin. Similarly, the binding of
donepezil hydrochloride to human albumin was not affected by furosemide,
digoxin and warfarin.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Mild to Moderate Alzheimer's Disease
The effectiveness of donepezil hydrochloride as a treatment for mild to
moderate Alzheimer's disease is demonstrated by the results of two randomized,
double-blind, placebo-controlled clinical investigations in patients with
Alzheimer's disease (diagnosed by NINCDS and DSM III-R criteria, Mini-Mental
State Examination ≥ 10 and ≤ 26 and Clinical Dementia Rating of 1 or 2). The
mean age of patients participating in donepezil hydrochloride trials was 73
years with a range of 50 to 94. Approximately 62% of patients were women and
38% were men. The racial distribution was white 95%, black 3% and other races
2%.
The higher dose of 10 mg did not provide a statistically significantly greater
clinical benefit than 5 mg. There is a suggestion, however, based upon order
of group mean scores and dose trend analyses of data from these clinical
trials, that a daily dose of 10 mg of donepezil hydrochloride might provide
additional benefit for some patients. Accordingly, whether or not to employ a
dose of 10 mg is a matter of prescriber and patient preference.
Study Outcome Measures
In each study, the effectiveness of treatment with donepezil hydrochloride was
evaluated using a dual outcome assessment strategy.
The ability of donepezil hydrochloride to improve cognitive performance was
assessed with the cognitive subscale of the Alzheimer's Disease Assessment
Scale (ADAS-cog), a multi-item instrument that has been extensively validated
in longitudinal cohorts of Alzheimer's disease patients. The ADAS-cog examines
selected aspects of cognitive performance including elements of memory,
orientation, attention, reasoning, language and praxis. The ADAS-cog scoring
range is from 0 to 70, with higher scores indicating greater cognitive
impairment. Elderly normal adults may score as low as 0 or 1, but it is not
unusual for non-demented adults to score slightly higher.
The patients recruited as participants in each study had mean scores on the
ADAS-cog of approximately 26 points, with a range from 4 to 61. Experience
based on longitudinal studies of ambulatory patients with mild to moderate
Alzheimer's disease suggest that scores on the ADAS-cog increase (worsen) by 6
to 12 points per year. However, smaller changes may be seen in patients with
very mild or very advanced disease since the ADAS-cog is not uniformly
sensitive to change over the course of the disease. The annualized rate of
decline in the placebo patients participating in donepezil hydrochloride
trials was approximately 2 to 4 points per year.
The ability of donepezil hydrochloride to produce an overall clinical effect
was assessed using a Clinician's Interview-Based Impression of Change that
required the use of caregiver information, the CIBIC-plus. The CIBIC-plus is
not a single instrument and is not a standardized instrument like the ADAS-
cog. Clinical trials for investigational drugs have used a variety of CIBIC
formats, each different in terms of depth and structure.
As such, results from a CIBIC-plus reflect clinical experience from the trial
or trials in which it was used and cannot be compared directly with the
results of CIBIC-plus evaluations from other clinical trials. The CIBIC-plus
used in donepezil hydrochloride trials was a semi-structured instrument that
was intended to examine four major areas of patient function: General,
Cognitive, Behavioral and Activities of Daily Living. It represents the
assessment of a skilled clinician based upon his/her observations at an
interview with the patient, in combination with information supplied by a
caregiver familiar with the behavior of the patient over the interval rated.
The CIBIC-plus is scored as a seven point categorical rating, ranging from a
score of 1, indicating "markedly improved," to a score of 4, indicating "no
change" to a score of 7, indicating "markedly worse." The CIBIC-plus has not
been systematically compared directly to assessments not using information
from caregivers (CIBIC) or other global methods.
Thirty-Week Study
In a study of 30 weeks duration, 473 patients were randomized to receive
single daily doses of placebo, 5 mg/day or 10 mg/day of donepezil
hydrochloride. The 30 week study was divided into a 24-week double-blind
active treatment phase followed by a 6-week single-blind placebo washout
period. The study was designed to compare 5 mg/day or 10 mg/day fixed doses of
donepezil hydrochloride to placebo. However, to reduce the likelihood of
cholinergic effects, the 10 mg/day treatment was started following an initial
7-day treatment with 5 mg/day doses.
Effects on the ADAS-cog
Figure 1 illustrates the time course for the change from baseline in ADAS-cog
scores for all three dose groups over the 30 weeks of the study. After 24
weeks of treatment, the mean differences in the ADAS-cog change scores for
donepezil hydrochloride treated patients compared to the patients on placebo
were 2.8 and 3.1 points for the 5 mg/day and 10 mg/day treatments,
respectively. These differences were statistically significant. While the
treatment effect size may appear to be slightly greater for the 10 mg/day
treatment, there was no statistically significant difference between the two
active treatments.
Following 6 weeks of placebo washout, scores on the ADAS-cog for both the
donepezil hydrochloride treatment groups were indistinguishable from those
patients who had received only placebo for 30 weeks. This suggests that the
beneficial effects of donepezil hydrochloride abate over 6 weeks following
discontinuation of treatment and do not represent a change in the underlying
disease. There was no evidence of a rebound effect 6 weeks after abrupt
discontinuation of therapy.
Figure 1. Time-course of the Change from Baseline in ADAS-cog Score for
Patients Completing 24 Weeks of Treatment
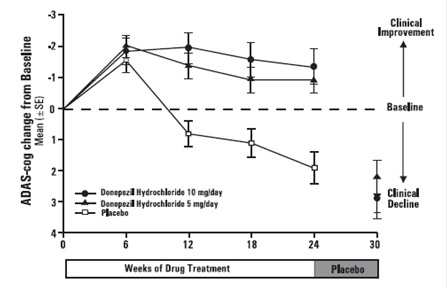
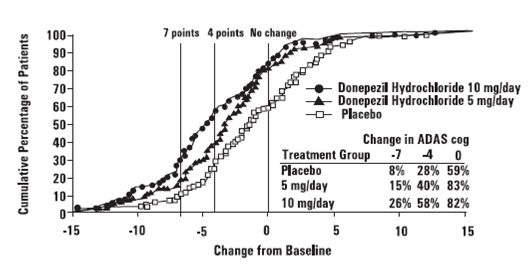
Figure 2 illustrates the cumulative percentages of patients from each of the
three treatment groups who had attained the measure of improvement in ADAS-cog
score shown on the X axis. Three change scores, (7-point and 4-point
reductions from baseline or no change in score) have been identified for
illustrative purposes, and the percent of patients in each group achieving
that result is shown in the inset table.
The curves demonstrate that both patients assigned to placebo and donepezil
hydrochloride have a wide range of responses, but that the active treatment
groups are more likely to show greater improvements. A curve for an effective
treatment would be shifted to the left of the curve for placebo, while an
ineffective or deleterious treatment would be superimposed upon or shifted to
the right of the curve for placebo.
Figure 2. Cumulative Percentage of Patients Completing 24 Weeks of Double-
blind Treatment with Specified Changes from Baseline ADAS-cog Scores. The
Percentages of Randomized Patients who Completed the Study were: Placebo 80%,
5 mg/day 85% and 10 mg/day 68%.
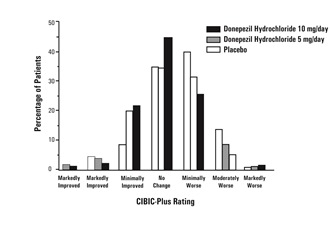
Effects on the CIBIC-plus
Figure 3 is a histogram of the frequency distribution of CIBIC-plus scores
attained by patients assigned to each of the three treatment groups who
completed 24 weeks of treatment. The mean drug-placebo differences for these
groups of patients were 0.35 points and 0.39 points for 5 mg/day and 10 mg/day
of donepezil hydrochloride, respectively. These differences were statistically
significant. There was no statistically significant difference between the two
active treatments.
Figure 3. Frequency Distribution of CIBIC plus Scores at Week 24.
Fifteen-Week Study
In a study of 15 weeks duration, patients were randomized to receive single
daily doses of placebo or either 5 mg/day or 10 mg/day of donepezil
hydrochloride for 12 weeks, followed by a 3-week placebo washout period. As in
the 30-week study, to avoid acute cholinergic effects, the 10 mg/day treatment
followed an initial 7-day treatment with 5 mg/day doses.
Effects on the ADAS-Cog
Figure 4 illustrates the time course of the change from baseline in ADAS-cog
scores for all three dose groups over the 15 weeks of the study. After 12
weeks of treatment, the differences in mean ADAS-cog change scores for the
donepezil hydrochloride treated patients compared to the patients on placebo
were 2.7 and 3.0 points each, for the 5 and 10 mg/day donepezil hydrochloride
treatment groups, respectively. These differences were statistically
significant. The effect size for the 10 mg/day group may appear to be slightly
larger than that for 5 mg/day. However, the differences between active
treatments were not statistically significant.
Figure 4. Time-course of the Change from Baseline in ADAS-cog Score for
Patients Completing the 15-week Study.
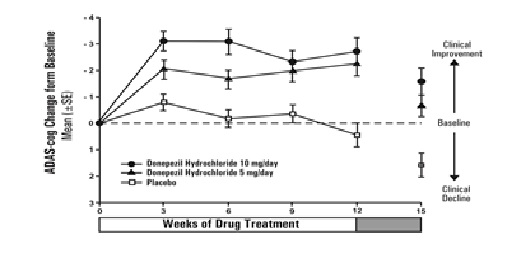
Patients Completing the 15-week Study.
Following 3 weeks of placebo washout, scores on the ADAS-cog for both the donepezil hydrochloride treatment groups increased, indicating that discontinuation of donepezil hydrochloride resulted in a loss of its treatment effect. The duration of this placebo washout period was not sufficient to characterize the rate of loss of the treatment effect, but, the 30-week study (see above) demonstrated that treatment effects associated with the use of donepezil hydrochloride abate within 6 weeks of treatment discontinuation.
Figure 5 illustrates the cumulative percentages of patients from each of the
three treatment groups who attained the measure of improvement in ADAS-cog
score shown on the X axis. The same three change scores, (7-point and 4-point
reductions from baseline or no change in score) as selected for the 30-week
study have been used for this illustration. The percentages of patients
achieving those results are shown in the inset table.
As observed in the 30-week study, the curves demonstrate that patients
assigned to either placebo or to donepezil hydrochloride have a wide range of
responses, but that the donepezil hydrochloride treated patients are more
likely to show greater improvements in cognitive performance.
Figure 5. Cumulative Percentage of Patients with Specified Changes from Baseline ADAS-cog Scores. The Percentage of Randomized Patients Within Each Treatment Group Who Completed the Study Were: Placebo 93 %, 5 mg/day 90% and 10 mg/day 82%
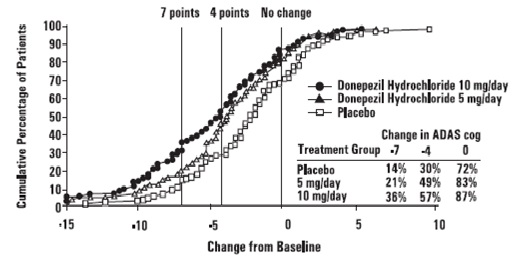
Effects on the CIBIC-plus
Figure 6 is a histogram of the frequency distribution of CIBIC-plus scores
attained by patients assigned to each of the three treatment groups who
completed 12 weeks of treatment. The differences in mean scores for donepezil
hydrochloride treated patients compared to the patients on placebo at Week 12
were 0.36 and 0.38 points for the 5 mg/day and 10 mg/day treatment groups,
respectively. These differences were statistically significant.
Figure 6. Frequency Distribution of CIBIC plus Scores at week 12
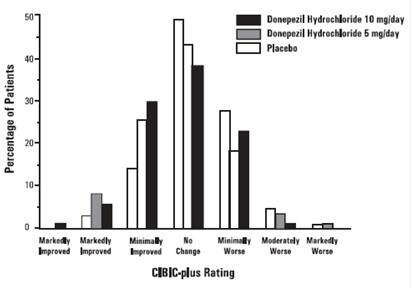
In both studies, patients age, sex and race were not found to predict the clinical outcome of donepezil hydrochloride treatment.
14.2 Severe Alzheimer's Disease
The effectiveness of donepezil hydrochloride in the treatment of patients with severe Alzheimer's Disease was established in studies employing dose of 10 mg/day.
Swedish 6 Month Study (10 mg/day)
The effectiveness of donepezil hydrochloride as a treatment for severe Alzheimer's disease is demonstrated by the results of a randomized, double- blind, placebo-controlled clinical study conducted in Sweden (6 month study) in patients with probable or possible Alzheimer's disease diagnosed by NINCDS- ADRDA and DSM-IV criteria, MMSE: range of 1 to 10. Two hundred and forty eight (248) patients with severe Alzheimer's disease were randomized to donepezil hydrochloride or placebo. For patients randomized to donepezil hydrochloride, treatment was initiated at 5 mg once daily for 28 days and then increased to 10 mg once daily. At the end of the 6 month treatment period, 90.5% of the donepezil hydrochloride treated patients were receiving the 10 mg/day dose. The mean age of patients was 84.9 years, with a range of 59 to 99. Approximately 77 % of patients were women, and 23 % were men. Almost all patients were Caucasian. Probable Alzheimer's disease was diagnosed in the majority of the patients (83.6% of donepezil hydrochloride treated patients and 84.2% of placebo treated patients).
Study Outcome Measures
The effectiveness of treatment with donepezil hydrochloride was determined
using a dual outcome assessment strategy that evaluated cognitive function
using an instrument designed for more impaired patients and overall function
through caregiver-rated assessment. This study showed that patients on
donepezil hydrochloride experienced significant improvement on both measures
compared to placebo.
The ability of donepezil hydrochloride to improve cognitive performance was assessed with the Severe Impairment Battery (SIB). The SIB, a multi-item instrument, has been validated for the evaluation of cognitive function in patients with moderate to severe dementia. The SIB evaluates selective aspects of cognitive performance, including elements of memory, language, orientation, attention, praxis, visuospatial ability, construction, and social interaction. The SIB scoring range is from 0 to 100, with lower scores indicating greater cognitive impairment.
Daily function was assessed using the Modified Alzheimer's Disease Cooperative Study Activities of Daily Living Inventory for Severe Alzheimer's Disease (ADCS-ADL-severe). The ADCS-ADL-severe is derived from the Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory, which is a comprehensive battery of ADL questions used to measure the functional capabilities of patients. Each ADL item is rated from the highest level of independent performance to complete loss. The ADCS-ADL-severe is a subset of 19 items, including ratings of the patient’s ability to eat, dress, bathe, use the telephone, get around (or travel), and perform other activities of daily living; it has been validated for the assessment of patients with moderate to severe dementia. The ADCS-ADL-severe has a scoring range of 0 to 54, with the lower scores indicating greater functional impairment. The investigator performs the inventory by interviewing a caregiver, in this study a nurse staff member, familiar with the functioning of the patient.
Effects on the SIB
Figure 7 shows the time course for the change from baseline in SIB score for the two treatment groups over the 6 months of the study. At 6 months of treatment, the mean difference in the SIB change scores for donepezil hydrochloride treated patients compared to patients on placebo was 5.9 points. Donepezil Hydrochloride treatment was statistically significantly superior to placebo.
Figure 7. Time Course of the Change from Baseline in SIB Score for
Patients Completing 6 months of Treatment.
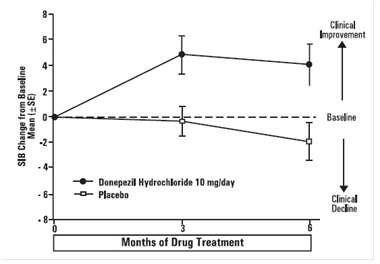
Figure 8 illustrates the cumulative percentages of patients from each of the
two treatment groups who attained the measure of improvement in SIB score
shown on the X-axis. While patients assigned both to donepezil hydrochloride
and to placebo have a wide range of responses, the curves show that the
donepezil hydrochloride group is more likely to show a greater improvement in
cognitive performance.
Figure 8. Cumulative Percentage of Patients Completing 6 Months of Double-
blind Treatment with Particular Changes from Baseline in SIB Scores.
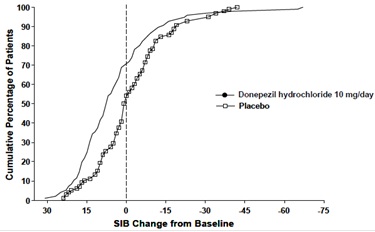
Figure 9. Time Course of the Change from Baseline in ADCS-ADL-Severe Score for Patients Completing 6 Months of Treatment.
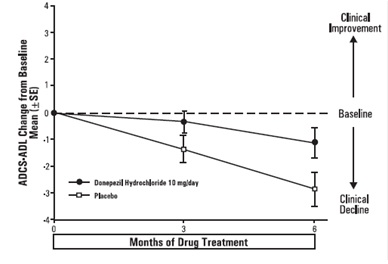
Effects on the ADCS-ADL-severe: Figure 9 illustrates the time course for
the change from baseline in ADCS-ADL-severe scores for patients in the two
treatment groups over the 6 months of the study. After 6 months of treatment,
the mean difference in the ADCS-ADL-severe change scores for donepezil
hydrochloride treated patients compared to patients on placebo was 1.8 points.
Donepezil hydrochloride treatment was statistically significantly superior to
placebo.
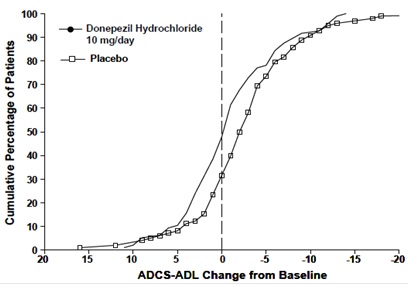
Figure 10 shows the cumulative percentages of patients from each treatment
group with specified changes from baseline ADCS-ADL-severe scores. While both
patients assigned to donepezil hydrochloride and placebo have a wide range of
responses, the curves demonstrate that the donepezil hydrochloride group is
more likely to show a smaller decline or an improvement.
Figure 10. Cumulative Percentage of Patients Completing 6 Months of Double- blind Treatment with Particular Changes from Baseline in ADCS-ADL-Severe Scores.
Japanese 24-Week Study (10 mg/day)
In a study of 24 weeks duration conducted in Japan, 325 patients with severe
Alzheimer's disease were randomized to doses of 5 mg/day or 10 mg/day of
donepezil, administered once daily, or placebo. Patients randomized to
treatment with donepezil were to achieve their assigned doses by titration,
beginning at 3 mg/day, and extending over a maximum
of 6 weeks. Two hundred and forty eight (248) patients completed the study,
with similar proportions of patients completing the study in each treatment
group. The primary efficacy measures for this study were the SIB and CIBIC-
plus.
At 24 weeks of treatment, statistically significant treatment differences were observed between the 10 mg/day dose of donepezil and placebo on both the SIB and CIBIC-plus. The 5 mg/day dose of donepezil showed a statistically significant superiority to placebo on the SIB, but not on the CIBIC-plus.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 Donepezil hydrochloride Tablets, USP
Donepezil hydrochloride tablets USP, 10 mg are yellow round biconvex, film coated tablets debossed with "I" on one side and "21" on the other side. They are supplied as follows
Bottles of 90 NDC 68071-3198-9
Storage: Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient
Information).
Instruct patients and caregivers to take donepezil hydrochloride tablets only
once per day, as prescribed.
Instruct patients and caregivers that donepezil hydrochloride tablets can be
taken with or without food.
Advise patients and caregivers that donepezil hydrochloride tablets may cause
nausea, diarrhea, insomnia, vomiting, muscle cramps, fatigue, and decreased
appetite.

Manufactured for:
Camber Pharmaceuticals Inc.
Piscataway, NJ 08854
Manufactured by:
HETERO TM****
HETERO LABS LIMITED2031825
Unit V, Polepally, Jadcherla,
Mahaboob Nagar-509 301, India.
Revised: September 2015
SPL UNCLASSIFIED SECTION
Patient Package Insert
Patient Package Insert
Donepezil Hydrochloride
(doe-NEP-e-zil HYE-droe-KLOR-ide) Tablets
Read the Patient Information that comes with donepezil hydrochloride tablets before the patient starts taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with the doctor about Alzheimer's disease or treatment for it. If you have questions, ask the doctor or pharmacist.
What is donepezil hydrochloride tablet?
Donepezil hydrochloride comes as donepezil hydrochloride film-coated tablets in dosage strengths of 5 mg and 10 mg.
Donepezil hydrochloride tablet is a prescription medicine to treat mild, moderate and severe Alzheimer's disease. Donepezil hydrochloride tablet can help with mental function and with doing daily tasks. Donepezil hydrochloride tablet does not work the same in all people. Some people may:
• Seem much better
• Get better in small ways or stay the same
• Get worse over time but slower than expected
• Not change and then get worse as expected
Donepezil hydrochloride tablets does not cure Alzheimer's disease. All patients with Alzheimer's disease get worse over time, even if they take donepezil hydrochloride tablets.
Donepezil hydrochloride tablets have not been approved as a treatment for any
medical condition in children.
Who should not take donepezil hydrochloride tablets?
The patient should not take donepezil hydrochloride tablets if allergic to any
of the ingredients in donepezil hydrochloride tablets or to medicines that
contain piperidines. Ask the patient's doctor if you are not sure. See the end
of this leaflet for a list of ingredients in donepezil hydrochloride tablets.
What should I tell the doctor before the patient takes donepezil
hydrochloride tablets?
Tell the doctor about all the patient's present or past health problems.
****Include:
• Any heart problems including problems with irregular, slow, or fast
heartbeats
• Asthma or lung problems
• A seizure
• Stomach ulcers
• Difficulty passing urine
• Liver or kidney problems
• Trouble swallowing tablets
• Present pregnancy or plans to become pregnant. It is not known if donepezil
hydrochloride tablets can harm an unborn baby.
• Present breast-feeding. It is not known if donepezil hydrochloride passes
into breast milk. Donepezil hydrochloride tablet is not for women who are
breast-feeding.
Tell the doctor about all the medicines the patient takes, including prescription and non-prescription medicines, vitamins, and herbal products. Donepezil Hydrochloride Tablets and other medicines may affect each other.
Be particularly sure to tell the doctor if the patient takes aspirin or
medicines called nonsteroidal anti-inflammatory drugs (NSAIDs). There are many
NSAID medicines, both prescription and non-prescription. Ask the doctor or
pharmacist if you are not sure if any of the patient's medicines are NSAIDs.
Taking NSAIDs and donepezil hydrochloride tablets together may make the
patient more likely to get stomach ulcers.
Donepezil hydrochloride tablets taken with certain medicines used for
anesthesia may cause side effects. Tell the responsible doctor or dentist that
the patient takes donepezil hydrochloride tablets before the patient has:
• surgery
• medical procedures
• dental surgery or procedures.
Know the medicines that the patient takes. Keep a list of all the patient's
medicines. Show it to the doctor or pharmacist before the patient starts a new
medicine.
How should the patient take donepezil hydrochloride tablets?
• Give donepezil hydrochloride tablets exactly as prescribed by the doctor. Do
not stop donepezil hydrochloride tablets or change the dose yourself. Talk
with the doctor first.
• Give donepezil hydrochloride tablets one time each day. Donepezil
hydrochloride tablets can be taken with or without food.
• If you miss giving the patient a dose of donepezil hydrochloride tablets,
just wait. Give only the next dose at the usual time. Do not give 2 doses at
the same time.
• If donepezil hydrochloride tablets are missed for 7 days or more, talk with
the doctor before starting again.
• If the patient takes too much donepezil hydrochloride tablets at one time,
call the doctor or poison control center, or go to the emergency room right
away.
What are the possible side effects of donepezil hydrochloride tablets?
Donepezil hydrochloride tablets may cause the following serious side
effects:
•slow heartbeat and fainting. This happens more often in people with
heart problems. Call the doctor right away if the patient faints while taking
donepezil hydrochloride tablets.
•more stomach acid. This raises the chance of ulcers and bleeding. The
risk is higher for patients who had ulcers, or take aspirin or other NSAIDs.
• worsening of lung problems in people with asthma or other lung disease.
• seizures.
• difficulty passing urine.
Call the doctorright away if the patient has:
• fainting.
• heartburn or stomach pain that is new or won't go away.
• nausea or vomiting, blood in the vomit, dark vomit that looks like coffee
grounds.
• bowel movements or stools that look like black tar.
• new or worse asthma or breathing problems.
• seizures.
• difficulty passing urine.
The most common side effects of donepezil hydrochloride tablets are:
• nausea
• diarrhea
• not sleeping well
• vomiting
• muscle cramps
• feeling tired
• not wanting to eat
These side effects may get better after the patient takes donepezil hydrochloride tablets for a while. This is not a complete list of side effects with donepezil hydrochloride tablets. For more information, ask the doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side
effects to FDA at 1-800-FDA-1088.
How should donepezil hydrochloride tablets be stored?
Store donepezil hydrochloride tablets at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Keep donepezil hydrochloride tablets and all medicines out of the reach of children.
General information about donepezil hydrochloride tablets
Medicines are sometimes prescribed for conditions that are not mentioned in this Patient Information Leaflet. Do not use donepezil hydrochloride tablets for a condition for which it was not prescribed. Do not give donepezil hydrochloride tablets to people other than the patient, even if they have the same symptoms as the patient, as it may harm them.
This leaflet summarizes the most important information about donepezil hydrochloride tablets. If you would like more information talk with the patient’s doctor. You can ask your pharmacist or doctor for information about donepezil hydrochloride that is written for health professionals.
What are the ingredients in donepezil hydrochloride tablets?
Active ingredient: donepezil hydrochloride USP
Inactive ingredients: corn starch, hydroxypropyl cellulose, lactose
monohydrate, magnesium stearate and microcrystalline cellulose. The film
coating contains hypromellose, polyethylene glycol, talc and titanium dioxide.
Additionally, the 10 mg tablet contains yellow iron oxide as a coloring agent.
Manufactured for:

Camber Pharmaceuticals Inc.
Piscataway, NJ 08854
Manufactured by:
HETERO TM
HETERO LABS LIMITED
Unit V, Polepally, Jadcherla,
Mahaboob Nagar-509 301, India.
Revised: September 2015
