Norepinephrine Bitartrate
These highlights do not include all the information needed to use NOREPINEPHRINE BITARTRATE INJECTION safely and effectively. See full prescribing information for NOREPINEPHRINE BITARTRATE INJECTION. Initial U.S. Approval: 1950
e375a0b9-4d26-4749-bbc3-43d63a7cf65a
HUMAN PRESCRIPTION DRUG LABEL
May 6, 2021
Baxter Healthcare Corporation
DUNS: 005083209
Baxter Healthcare Company
DUNS: 005083209
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
norepinephrine bitartrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
DESCRIPTION SECTION
11 DESCRIPTION
Norepinephrine (sometimes referred to as l-arterenol/Levarterenol or l-norepinephrine) is a sympathomimetic amine which differs from epinephrine by the absence of a methyl group on the nitrogen atom.
Norepinephrine Bitartrate is (-)-α-(aminomethyl)-3,4-dihydroxybenzyl alcohol tartrate (1:1) (salt) monohydrate (molecular weight 337.3 g/mol) and has the following structural formula:
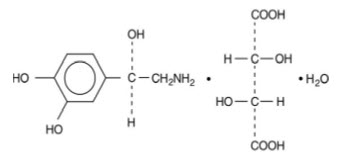
Norepinephrine Bitartrate Injection, USP is supplied in a sterile aqueous solution in the form of the bitartrate salt to be administered by intravenous infusion. Norepinephrine is sparingly soluble in water, very slightly soluble in alcohol and ether, and readily soluble in acids. Each mL contains 1 mg of norepinephrine base (equivalent to 1.89 mg of norepinephrine bitartrate, anhydrous basis), sodium chloride for isotonicity, not more than 0.2 mg (vials) of sodium metabisulfite as an antioxidant. It has a pH of 3.0 to 4.5. The air in the containers has been displaced by nitrogen gas.
