Tartar control plus
Equate 855.000/855AA-AC Tartar Control Plus Antiseptic Mouth Rinse, Blue Mint
4da4ecf6-038e-4a64-83f7-c29d70e83174
HUMAN OTC DRUG LABEL
May 12, 2025
Walmart Inc.
DUNS: 051957769
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Eucalyptol, menthol, methyl salicylate, thymol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (14)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
principal display panel
NDC 49055-855-12
equate ™
Compare to Listerine ® Ultraclean ® Cool Mint ® Antiseptic Mouthwash active ingredients*
Tartar Control Plus Mputhwash
Antiseptic Mouth Rinse Plus
Antigingivitis/Antiplaque
Blue Mint
Long lasting clean feel
Controls tartar that can discolor teeth
Fresh breath with mouth-cooling sensation
Made in the USA with domestic and imported ingredients
Factory Certified
IMPORTANT: Read directions for proper use.
50.7 FL OZ (1.58 QT) 1.5 L

INDICATIONS & USAGE SECTION
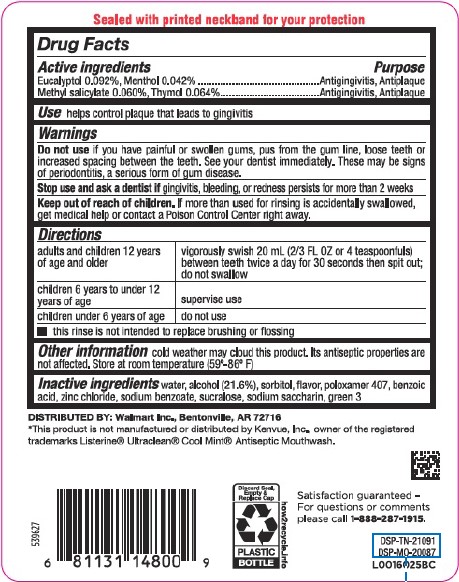
Use
helps control plaque that leads to gingivitis
ADVERSE REACTIONS SECTION
Adverse Reactions
DISTRIBUTED BY: Walmart Inc., Bentonville, AR 72716
Satisfaction guaranteed - For questions or comments please call 1-888-287-1915.
Discard Seal, Empty & Replace Cap
PLASTIC BOTTLE
how2recycle.info
DSP-TN-21091
DSP-MO-20087
INACTIVE INGREDIENT SECTION
Inactive ingredients
water, alcohol (21.6%), sorbitol, flavor, poloxamer 407, benzoic acid, zinc chloride, sodium benzoate, sucralose, sodium saccharin, green 3
OTHER SAFETY INFORMATION
Tamper Evident Statement
Sealed with printed neckband for your protection
OTC - ACTIVE INGREDIENT SECTION
Active ingredients
Eucalyptol 0.092%
Menthol 0.042%
Methyl salicylate 0.060%
Thymol 0.064%
OTC - PURPOSE SECTION
Purpose
Antigingivitis, antiplaque
WARNINGS SECTION
Warnings
For this product
OTC - DO NOT USE SECTION
Do not use
if you have painful or swollen gums, pus from the gum line, loose teeth or increased spacing between the teeth. See your dentist immediately. These may be signs of periodontitis, a serious form of gum disease.
OTC - STOP USE SECTION
Stop use and ask a dentist if
gingivitis, bleeding, or redness persists for more than 2 weeks.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children.
If more than used for rinsing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
adults and children 12 years of age and older - vigorously swish 20 mL (2/3 FL OZ or 4 teaspoonfuls) between teeth twice a day for 30 seconds then spit out; do not swallow
children 6 years to under 12 years of age - supervise use
children under 6 years of age - do not use
- this rinse is not intended to replace brushing or flossing
STORAGE AND HANDLING SECTION
Other information
cold weather may cloud this product. Its antiseptic properties are not affected. Store at room temperature (59°-86°F)
SPL UNCLASSIFIED SECTION
Disclaimer
*This product is not manufactured or distributed by Kenvue, Inc., owner of the registered trademarks Listerine ® Ultraclean ® Cool Mint ® Antiseptic Mouthwash.
