DOXEPIN HYDROCHLORIDE
Doxepin Hydrochloride Capsules, USP
d7228639-25fa-4311-9241-11ad6af5c5e2
HUMAN PRESCRIPTION DRUG LABEL
Sep 8, 2025
NORTHSTAR RX LLC
DUNS: 830546433
Products 5
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
DOXEPIN HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (14)
DOXEPIN HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (14)
DOXEPIN HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (14)
DOXEPIN HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (14)
DOXEPIN HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (14)
Drug Labeling Information
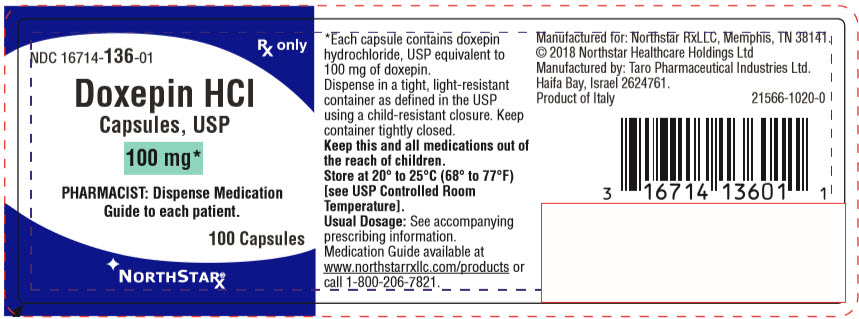
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 100 mg Capsule Bottle Label
NDC 16714-136-01
Rx only
Doxepin HCl
Capsules, USP
100 mg*
PHARMACIST: Dispense Medication
Guide to each patient.
100 Capsules
NORTHSTARx ®
BOXED WARNING SECTION
Suicidality and Antidepressant Drugs
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents and young adults in short- term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of doxepin or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Doxepin is not approved for use in pediatric patients. (SeeWARNINGS: Clinical Worsening and Suicide Risk,PRECAUTIONS: Information for PatientsandPRECAUTIONS: Pediatric Use.)
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
**NOTE:**Some of the adverse reactions noted below have not been specifically reported with doxepin use. However, due to the close pharmacological similarities among the tricyclics, the reactions should be considered when prescribing doxepin.
**Anticholinergic Effects:**Dry mouth, blurred vision, constipation and urinary retention have been reported. If they do not subside with continued therapy or become severe, it may be necessary to reduce the dosage.
**Central Nervous System Effects:**Drowsiness is the most commonly noticed side effect. This tends to disappear as therapy is continued. Other infrequently reported CNS side effects are confusion, disorientation, hallucinations, numbness, paresthesias, ataxia, extrapyramidal symptoms, seizures, tardive dyskinesia and tremor.
**Cardiovascular:**Cardiovascular effects including hypotension, hypertension and tachycardia have been reported occasionally.
**Allergic:**Skin rash, edema, photosensitization and pruritus have occasionally occurred.
**Hematologic:**Eosinophilia has been reported in a few patients. There have been occasional reports of bone marrow depression manifesting as agranulocytosis, leukopenia, thrombocytopenia and purpura.
**Gastrointestinal:**Nausea, vomiting, indigestion, taste disturbances, diarrhea, anorexia and aphthous stomatitis have been reported. (See Anticholinergic Effects.)
**Endocrine:**Raised or lowered libido, testicular swelling, gynecomastia in males, enlargement of breasts and galactorrhea in the female, raising or lowering of blood sugar levels and syndrome of inappropriate antidiuretic hormone secretion have been reported with tricyclic administration.
**Other:**Dizziness, tinnitus, weight gain, sweating, chills, fatigue, weakness, flushing, jaundice, alopecia, headache, exacerbation of asthma, angle closure glaucoma, mydriasis and hyperpyrexia (in association with chlorpromazine) have been occasionally observed as adverse effects.
Withdrawal Symptoms
The possibility of development of withdrawal symptoms upon abrupt cessation of treatment after prolonged doxepin administration should be borne in mind. These are not indicative of addiction and gradual withdrawal of medication should not cause these symptoms.
HOW SUPPLIED SECTION
HOW SUPPLIED
Doxepin Hydrochloride Capsules, USP are available containing doxepin hydrochloride, USP equivalent to 10 mg, 25 mg, 50 mg, 75 mg or 100 mg of doxepin.
The 10 mg capsule is a hard-shell, gelatin capsule with a tan opaque cap and
tan opaque body, axially printed withTAROoverD10in black ink on both
the cap and the body. They are available as follows:
NDC 16714-132-01 bottles of 100 capsules
The 25 mg capsule is a hard-shell, gelatin capsule with a yellow opaque cap
and white opaque body, axially printed withTAROoverD25in black ink
on both the cap and the body. They are available as follows:
NDC 16714-133-01 bottles of 100 capsules
The 50 mg capsule is a hard-shell, gelatin capsule with a yellow opaque cap
and yellow opaque body, axially printed withTAROoverD50in black ink
on both the cap and the body. They are available as follows:
NDC 16714-134-01 bottles of 100 capsules
The 75 mg capsule is a hard-shell, gelatin capsule with a green opaque cap and
green opaque body, axially printed withTAROoverD75in black ink on
both the cap and the body. They are available as follows:
NDC 16714-135-01 bottles of 100 capsules
The 100 mg capsule is a hard-shell, gelatin capsule with a green opaque cap
and white opaque body, axially printed withTAROoverD100in black ink
on both the cap and the body. They are available as follows:
NDC 16714-136-01 bottles of 100 capsules
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Protect from light.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
DESCRIPTION SECTION
DESCRIPTION
Doxepin hydrochloride is one of a class of psychotherapeutic agents known as dibenzoxepin tricyclic compounds. The molecular formula of the compound is C 19H 21NO ∙ HCl having a molecular weight of 315.84. It is a white crystalline powder freely soluble in water, in ethanol (96%), and methylene chloride. It may be represented by the following structural formula:
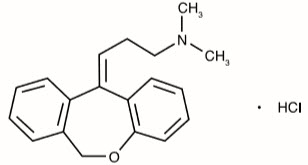
Chemically, doxepin hydrochloride is a dibenzoxepin derivative and is the first of a family of tricyclic psychotherapeutic agents. Specifically, it is an isomeric mixture of 1-Propanamine, 3-dibenz[ b,e] oxepin-11 (6 H)ylidene- N,N-dimethyl-hydrochloride.
Each 10 mg, 25 mg, 50 mg, 75 mg and 100 mg doxepin capsule for oral administration contains doxepin hydrochloride, USP equivalent to 10 mg, 25 mg, 50 mg, 75 mg and 100 mg of doxepin, respectively and the following inactive ingredients: colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, pregelatinized starch and sodium lauryl sulfate. The empty gelatin capsule shells contain gelatin, sodium lauryl sulfate and titanium dioxide. In addition, the 10 mg empty gelatin capsule shells contain iron oxide red and iron oxide yellow, the 25 mg and 50 mg empty gelatin capsule shells contain D&C Yellow 10 and FD&C Yellow 6, and the 75 mg and 100 mg empty gelatin capsule shells contain D&C Yellow 10 and FD&C Blue 1.
The imprinting ink may contain ferrosoferric oxide, potassium hydroxide, propylene glycol and shellac glaze.
SPL UNCLASSIFIED SECTION
Manufactured for: Northstar RxLLC, Memphis, TN 38141.
Manufactured by: Taro Pharmaceutical Industries Ltd., Haifa Bay, Israel
2624761.
Medication Guide available at www.northstarrxllc.com/productsor call
1-800-206-7821.
Issued: October 2020
21561-1020-0
WARNINGS SECTION
WARNINGS
Clinical Worsening and Suicide Risk
Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18 to 24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
The pooled analyses of placebo-controlled trials in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of nine antidepressant drugs in over 4,400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs. placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1,000 patients treated) are provided in Table 1.
Table 1|
Age Range |
Drug-Placebo Difference in Number of Cases of Suicidality Per 1,000 Patients Treated |
|---|---|
|
Increases Compared to Placebo | |
|
< 18 |
14 additional cases |
|
18 to 24 |
5 additional cases |
|
Decreases Compared to Placebo | |
|
25 to 64 |
1 fewer case |
|
**≥**65 |
6 fewer cases |
No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about drug effect on suicide.
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo- controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient's presenting symptoms.
**Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to healthcare providers. Such monitoring should include daily observation by families and caregivers.**Prescriptions for doxepin should be written for the smallest number of capsules consistent with good patient management, in order to reduce the risk of overdose.
Screening Patients for Bipolar Disorder
A major depressive episode may be the initial presentation of bipolar disorder. It is generally believed (though not established in controlled trials) that treating such an episode with an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder. Whether any of the symptoms described above represent such a conversion is unknown. However, prior to initiating treatment with an antidepressant, patients with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression. It should be noted that doxepin is not approved for use in treating bipolar depression.
Angle-Closure Glaucoma
The pupillary dilation that occurs following use of many antidepressant drugs including doxepin hydrochloride capsules may trigger an angle closure attack in a patient with anatomically narrow angles who does not have a patent iridectomy.
Geriatric Use
The use of doxepin on a once a day dosage regimen in geriatric patients should be adjusted carefully based on the patient's condition (see PRECAUTIONS: Geriatric Use).
Pregnancy
Reproduction studies have been performed in rats, rabbits, monkeys and dogs and there was no evidence of harm to the animal fetus. The relevance to humans is not known. Since there is no experience in pregnant women who have received this drug, safety in pregnancy has not been established. There has been a report of apnea and drowsiness occurring in a nursing infant whose mother was taking doxepin.
Pediatric Use
The use of doxepin in children under 12 years of age is not recommended because safe conditions for its use have not been established.
PRECAUTIONS SECTION
PRECAUTIONS
Information for Patients
Prescribers or other health professionals should inform patients, their families and their caregivers about the benefits and risks associated with treatment with doxepin and should counsel them in its appropriate use. A patient Medication Guide about "Antidepressant Medicines, Depression and Other Serious Mental Illnesses and Suicidal Thoughts or Actions" is available for doxepin. The prescriber or health professional should instruct patients, their families and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document.
Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking doxepin.
Clinical Worsening and Suicide Risk
Patients, their families and their caregivers should be encouraged to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, mania, other unusual changes in behavior, worsening of depression and suicidal ideation, especially early during antidepressant treatment and when the dose is adjusted up or down. Families and caregivers of patients should be advised to look for the emergence of such symptoms on a day to day basis, since changes may be abrupt. Such symptoms should be reported to the patient's prescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patient's presenting symptoms. Symptoms such as these may be associated with an increased risk for suicidal thinking and behavior and indicate a need for very close monitoring and possibly changes in the medication.
Patients should be advised that taking doxepin hydrochloride capsules can cause mild pupillary dilation, which in susceptible individuals, can lead to an episode of angle-closure glaucoma. Pre-existing glaucoma is almost always open-angle glaucoma because angle-closure glaucoma, when diagnosed, can be treated definitively with iridectomy. Open-angle glaucoma is not a risk factor for angle-closure glaucoma. Patients may wish to be examined to determine whether they are susceptible to angle closure, and have a prophylactic procedure (e.g., iridectomy), if they are susceptible.
Drug Interactions
Drugs Metabolized by P450 2D6
The biochemical activity of the drug metabolizing isozyme cytochrome P450 2D6 (debrisoquin hydroxylase) is reduced in a subset of the Caucasian population (about 7% to 10% of Caucasians are so called "poor metabolizers"); reliable estimates of the prevalence of reduced P450 2D6 isozyme activity among Asian, African and other populations are not yet available. Poor metabolizers have higher than expected plasma concentrations of tricyclic antidepressants (TCAs) when given usual doses. Depending on the fraction of drug metabolized by P450 2D6, the increase in plasma concentration may be small, or quite large (8-fold increase in plasma AUC of the TCA).
In addition, certain drugs inhibit the activity of this isozyme and make normal metabolizers resemble poor metabolizers. An individual who is stable on a given dose of TCA may become abruptly toxic when given one of these inhibiting drugs as concomitant therapy. The drugs that inhibit cytochrome P450 2D6 include some that are not metabolized by the enzyme (quinidine; cimetidine) and many that are substrates for P450 2D6 (many other antidepressants, phenothiazines, and the Type 1C antiarrhythmics propafenone and flecainide). While all the selective serotonin reuptake inhibitors (SSRIs), e.g., citalopram, escitalopram, fluoxetine, sertraline, and paroxetine, inhibit P450 2D6, they may vary in the extent of inhibition. The extent to which SSRI-TCA interactions may pose clinical problems will depend on the degree of inhibition and the pharmacokinetics of the SSRI involved. Nevertheless, caution is indicated in the coadministration of TCAs with any of the SSRIs and also in switching from one class to the other. Of particular importance, sufficient time must elapse before initiating TCA treatment in a patient being withdrawn from fluoxetine, given the long half-life of the parent and active metabolite (at least 5 weeks may be necessary).
Concomitant use of tricyclic antidepressants with drugs that can inhibit cytochrome P450 2D6 may require lower doses than usually prescribed for either the tricyclic antidepressant or the other drug. Furthermore, whenever one of these other drugs is withdrawn from cotherapy, an increased dose of tricyclic antidepressant may be required. It is desirable to monitor TCA plasma levels whenever a TCA is going to be coadministered with another drug known to be an inhibitor of P450 2D6.
Doxepin is primarily metabolized by CYP2D6 (with CYP1A2 & CYP3A4 as minor pathways). Inhibitors or substrates of CYP2D6 (i.e., quinidine, selective serotonin reuptake inhibitors [SSRIs]) may increase the plasma concentration of doxepin when administered concomitantly. The extent of interaction depends on the variability of effect on CYP2D6. The clinical significance of this interaction with doxepin has not been systematically evaluated.
MAO Inhibitors
Serious side effects and even death have been reported following the concomitant use of certain drugs with MAO inhibitors. Therefore, MAO inhibitors should be discontinued at least 2 weeks prior to the cautious initiation of therapy with doxepin. The exact length of time may vary and is dependent upon the particular MAO inhibitor being used, the length of time it has been administered, and the dosage involved.
Cimetidine
Cimetidine has been reported to produce clinically significant fluctuations in steady-state serum concentrations of various tricyclic antidepressants. Serious anticholinergic symptoms (i.e., severe dry mouth, urinary retention and blurred vision) have been associated with elevations in the serum levels of tricyclic antidepressant when cimetidine therapy is initiated. Additionally, higher than expected tricyclic antidepressant levels have been observed when they are begun in patients already taking cimetidine. In patients who have been reported to be well controlled on tricyclic antidepressants receiving concurrent cimetidine therapy, discontinuation of cimetidine has been reported to decrease established steady-state serum tricyclic antidepressant levels and compromise their therapeutic effects.
Alcohol
It should be borne in mind that alcohol ingestion may increase the danger inherent in any intentional or unintentional doxepin overdosage. This is especially important in patients who may use alcohol excessively.
Tolazamide
A case of severe hypoglycemia has been reported in a type II diabetic patient maintained on tolazamide (1 gm/day) 11 days after the addition of doxepin (75 mg/day).
Drowsiness
Since drowsiness may occur with the use of this drug, patients should be warned of the possibility and cautioned against driving a car or operating dangerous machinery while taking the drug. Patients should also be cautioned that their response to alcohol may be potentiated.
Sedating drugs may cause confusion and over sedation in the elderly; elderly patients generally should be started on low doses of doxepin and observed closely (see PRECAUTIONS: Geriatric Use).
Suicide
Since suicide is an inherent risk in any depressed patient and may remain so until significant improvement has occurred, patients should be closely supervised during the early course of therapy. Prescriptions should be written for the smallest feasible amount.
Psychosis
Should increased symptoms of psychosis or shift to manic symptomatology occur, it may be necessary to reduce dosage or add a major tranquilizer to the dosage regimen.
Pediatric Use
Safety and effectiveness in the pediatric population have not been established (see BOX WARNINGand WARNINGS: Clinical Worsening and Suicide Risk).
Anyone considering the use of doxepin in a child or adolescent must balance the potential risks with the clinical need.
Geriatric Use
A determination has not been made whether controlled clinical studies of doxepin included sufficient numbers of subjects aged 65 and over to define a difference in response from younger subjects.
Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
The extent of renal excretion of doxepin has not been determined. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selections.
Sedating drugs may cause confusion and over sedation in the elderly; elderly patients generally should be started on low doses of doxepin and observed closely. (See WARNINGS.)
OVERDOSAGE SECTION
OVERDOSAGE
Deaths may occur from overdosage with this class of drugs. Multiple drug ingestion (including alcohol) is common in deliberate tricyclic antidepressant overdose. As the management is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment. Signs and symptoms of toxicity develop rapidly after tricyclic antidepressant overdose; therefore, hospital monitoring is required as soon as possible.
Manifestations
Critical manifestations of overdose include: cardiac dysrhythmias, severe hypotension, convulsions and CNS depression, including coma. Changes in the electrocardiogram, particularly in QRS axis or width, are clinically significant indicators of tricyclic antidepressant toxicity.
Other signs of overdose may include: confusion, disturbed concentration, transient visual hallucinations, dilated pupils, agitation, hyperactive reflexes, stupor, drowsiness, muscle rigidity, vomiting, hypothermia, hyperpyrexia, or any of the symptoms listed under ADVERSE REACTIONS.
Deaths have been reported involving overdoses of doxepin.
General Recommendations
General
Obtain an ECG and immediately initiate cardiac monitoring. Protect the patient's airway, establish an intravenous line and initiate gastric decontamination. A minimum of 6 hours of observation with cardiac monitoring and observation for signs of CNS or respiratory depression, hypotension, cardiac dysrhythmias and/or conduction blocks, and seizures is strongly advised. If signs of toxicity occur at any time during this period, extended monitoring is recommended. There are case reports of patients succumbing to fatal dysrhythmias late after overdose; these patients had clinical evidence of significant poisoning prior to death and most received inadequate gastrointestinal decontamination. Monitoring of plasma drug levels should not guide management of the patient.
Gastrointestinal Decontamination
All patients suspected of tricyclic antidepressant overdose should receive gastrointestinal decontamination. This should include large volume gastric lavage followed by activated charcoal. If consciousness is impaired, the airway should be secured prior to lavage. Emesis is contraindicated.
Cardiovascular
A maximal limb lead QRS duration of ≥ 0.10 seconds may be the best indication of the severity of the overdose. Intravenous sodium bicarbonate should be used to maintain the serum pH in the range of 7.45 to 7.55. If the pH response is inadequate, hyperventilation may also be used. Concomitant use of hyperventilation and sodium bicarbonate should be done with extreme caution, with frequent pH monitoring. A pH > 7.60 or a pCO 2< 20 mm Hg is undesirable. Dysrhythmias unresponsive to sodium bicarbonate therapy/hyperventilation may respond to lidocaine, bretylium or phenytoin. Type 1A and 1C antiarrhythmics are generally contraindicated (e.g., quinidine, disopyramide and procainamide).
In rare instances, hemoperfusion may be beneficial in acute refractory cardiovascular instability in patients with acute toxicity. However, hemodialysis, peritoneal dialysis, exchange transfusions and forced diuresis generally have been reported as ineffective in tricyclic antidepressant poisoning.
CNS
In patients with CNS depression, early intubation is advised because of the potential for abrupt deterioration. Seizures should be controlled with benzodiazepines, or if these are ineffective, other anticonvulsants (e.g., phenobarbital, phenytoin). Physostigmine is not recommended except to treat life threatening symptoms that have been unresponsive to other therapies, and then only in consultation with a poison control center.
Psychiatric Follow-up
Since overdosage is often deliberate, patients may attempt suicide by other means during the recovery phase. Psychiatric referral may be appropriate.
Pediatric Management
The principles of management of child and adult overdosages are similar. It is strongly recommended that the physician contact the local poison control center for specific pediatric treatment.
SPL MEDGUIDE SECTION
Medication Guide
Antidepressant Medicines, Depression and Other Serious Mental Illnesses, and Suicidal Thoughts or Actions
Read the Medication Guide that comes with your or your family member's
antidepressant medicine. This Medication Guide is only about the risk of
suicidal thoughts and actions with antidepressant medicines.
Talk to your, or your family member's, healthcare provider about:
- all risks and benefits of treatment with antidepressant medicines
- all treatment choices for depression or other serious mental illness
What is the most important information I should know about antidepressant
medicines, depression and other serious mental illnesses, and suicidal
thoughts or actions?
1. Antidepressant medicines may increase suicidal thoughts or actions in
some children, teenagers, and young adults within the first few months of
treatment.
**2. Depression and other serious mental illnesses are the most important
causes of suicidal thoughts and actions. Some people may have a particularly
high risk of having suicidal thoughts or actions.**These include people who
have (or have a family history of) bipolar illness (also called manic-
depressive illness) or suicidal thoughts or actions.
3. How can I watch for and try to prevent suicidal thoughts and actions in
myself or a family member?
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
|
|
What else do I need to know about antidepressant medicines?
***Never stop an antidepressant medicine without first talking to a healthcare provider.**Stopping an antidepressant medicine suddenly can cause other symptoms. ***Visual problems:**Only some people are at risk for these problems. You may want to undergo an eye examination to see if you are at risk and receive preventative treatment if you are. ***Antidepressants are medicines used to treat depression and other illnesses.**It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants. ***Antidepressant medicines have other side effects.**Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member. ***Antidepressant medicines can interact with other medicines.**Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider. ***Not all antidepressant medicines prescribed for children are FDA approved for use in children.**Talk to your child's healthcare provider for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
This Medication Guide has been approved by the U.S. Food and Drug Administration for all antidepressants.
Medication Guide available at www.northstarrxllc.com/productsor call
1-800-206-7821.
Manufactured for: Northstar RxLLC, Memphis, TN 38141.
Manufactured by: Taro Pharmaceutical Industries Ltd., Haifa Bay, Israel
2624761.
Issued: October 2020
21561-1020-0
