ONEXTON
These highlights do not include all the information needed to use ONEXTON Gel safely and effectively. See full prescribing information for ONEXTON Gel. ONEXTON® (clindamycin phosphate and benzoyl peroxide) gel, 1.2%/3.75% for topical use Initial U.S. Approval: 2000
697e760a-c206-4565-8f4e-a8085e215827
HUMAN PRESCRIPTION DRUG LABEL
Jul 23, 2025
Bausch Health US, LLC
DUNS: 831922468
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Clindamycin Phosphate and Benzoyl Peroxide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 50 gram Carton
NDC 0187-3050-50
Rx only
ONEXTON®
(Clindamycin Phosphate and
Benzoyl Peroxide) Gel, 1.2%/3.75%
For Topical Use Only
Not for ophthalmic, oral or intravaginal use
KEEP OUT OF REACH OF CHILDREN
One premixed 50-gram pump dispenser
Ortho Dermatologics

INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
ONEXTON® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/3.75% is indicated for the topical treatment of acne vulgaris in patients 12 years of age and older.
ONEXTON Gel is a combination of clindamycin phosphate (a lincosamide antibacterial) and benzoyl peroxide indicated for the topical treatment of acne vulgaris in patients 12 years of age and older. (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
4.1 Hypersensitivity
ONEXTON Gel is contraindicated in those individuals who have shown hypersensitivity to clindamycin, benzoyl peroxide, any components of the formulation, or lincomycin. Anaphylaxis, as well as allergic reactions leading to hospitalization, has been reported in postmarketing use with ONEXTON Gel [see Postmarketing Experience (6.2)].
4.2 Colitis/Enteritis
ONEXTON Gel is contraindicated in patients with a history of regional enteritis, ulcerative colitis, or antibiotic-associated colitis [see Warnings and Precautions (5.1)].
ONEXTON Gel is contraindicated in:
•
Patients who have demonstrated hypersensitivity (e.g., anaphylaxis) to clindamycin, benzoyl peroxide, any components of the formulation, or lincomycin. (4.1)
•
Patients with a history of regional enteritis, ulcerative colitis, or antibiotic-associated colitis. (4.2)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Colitis
Systemic absorption of clindamycin has been demonstrated following topical use of clindamycin. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been reported with the use of topical and systemic clindamycin. If significant diarrhea occurs, ONEXTON Gel should be discontinued.
Severe colitis has occurred following oral and parenteral administration of clindamycin with an onset of up to several weeks following cessation of therapy. Antiperistaltic agents such as opiates and diphenoxylate with atropine may prolong and/or worsen severe colitis. Severe colitis may result in death.
Studies indicate toxin(s) produced by Clostridia is one primary cause of antibiotic-associated colitis. The colitis is usually characterized by severe persistent diarrhea and severe abdominal cramps and may be associated with the passage of blood and mucus. Stool cultures for Clostridium difficile and stool assay for C. difficile toxin may be helpful diagnostically.
5.2 Ultraviolet Light and Environmental Exposure
Minimize sun exposure (including use of tanning beds or sun lamps) following drug application.
5.3 Concomitant Topical Medications
Concomitant topical acne therapy should be used with caution since a possible cumulative irritancy effect may occur, especially with the use of peeling, desquamating, or abrasive agents. If irritancy or dermatitis occurs, reduce frequency of application or temporarily interrupt treatment and resume once the irritation subsides. Treatment should be discontinued if the irritation persists.
•
Colitis: Clindamycin can cause severe colitis, which may result in death. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been reported with the use of clindamycin. ONEXTON Gel should be discontinued if significant diarrhea occurs. (5.1)
•
Ultraviolet Light and Environmental Exposure: Minimize sun exposure following drug application. (5.2)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following adverse reaction is described in more detail in the Warnings and Precautions section of the label:
•
Colitis [see Warnings and Precautions (5.1)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates observed in clinical trials of another drug and may not reflect the rates observed in clinical practice.
These adverse reactions occurred in less than 0.5% of subjects treated with ONEXTON Gel: burning sensation (0.4%); contact dermatitis (0.4%); pruritus (0.4%); and rash (0.4%).
During the clinical trial, subjects were assessed for local cutaneous signs and symptoms of erythema, scaling, itching, burning and stinging. Most local skin reactions either were the same as baseline or increased and peaked around Week 4 and were near or improved from baseline levels by Week 12. The percentage of subjects that had symptoms present before treatment (at baseline), during treatment, and the percent with symptoms present at Week 12 are shown in Table 1.
Table 1: Percent of Subjects with Local Skin Reactions. Results from the Phase 3 Trial (N = 243)|
Before Treatment |
During |
End of Treatment | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
Mild |
Mod.***** |
Severe |
Mild |
Mod.***** |
Severe |
Mild |
Mod.***** |
Severe | |
|
Erythema |
20 |
6 |
0 |
28 |
5 |
<1 |
15 |
2 |
0 |
|
Scaling |
10 |
1 |
0 |
19 |
3 |
0 |
10 |
<1 |
0 |
|
Itching |
14 |
3 |
<1 |
15 |
3 |
0 |
7 |
2 |
0 |
|
Burning |
5 |
<1 |
<1 |
7 |
1 |
<1 |
3 |
<1 |
0 |
|
Stinging |
5 |
<1 |
0 |
7 |
0 |
<1 |
3 |
0 |
<1 |
*Mod. = Moderate
6.2 Postmarketing Experience
Because postmarketing adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Anaphylaxis, as well as allergic reactions leading to hospitalizations, has been reported in postmarketing use of products containing clindamycin phosphate/benzoyl peroxide.
The most common adverse reactions are: burning sensation (0.4%); contact dermatitis (0.4%); pruritus (0.4%); and rash (0.4%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bausch Health US, LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Erythromycin
Avoid using ONEXTON Gel in combination with topical or oral erythromycin- containing products due to its clindamycin component. In vitro studies have shown antagonism between erythromycin and clindamycin. The clinical significance of this in vitro antagonism is not known.
7.2 Neuromuscular Blocking Agents
Clindamycin has been shown to have neuromuscular blocking properties that may enhance the action of other neuromuscular blocking agents. ONEXTON Gel should be used with caution in patients receiving such agents.
Avoid using ONEXTON Gel in combination with topical or oral erythromycin- containing products because of its clindamycin component. (7.1)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
Before applying ONEXTON Gel, wash the face gently with a mild soap, rinse with warm water, and pat the skin dry. Apply a pea-sized amount of ONEXTON Gel to the face once daily. Avoid the eyes, mouth, mucous membranes, or areas of broken skin.
Use of ONEXTON Gel beyond 12 weeks has not been evaluated.
ONEXTON Gel is not for oral, ophthalmic, or intravaginal use.
•
Apply a pea-sized amount of ONEXTON Gel to the face once daily. (2)
•
Not for oral, ophthalmic, or intravaginal use. (2)
SPL PATIENT PACKAGE INSERT SECTION
Patient Package Insert
[Enter Patient Package Insert here]
DESCRIPTION SECTION
11 DESCRIPTION
ONEXTON Gel is a combination product with two active ingredients in a white to off-white, opaque, smooth, aqueous gel formulation intended for topical use. Clindamycin phosphate is a water-soluble ester of the semisynthetic antibiotic produced by a 7(S)-chloro-substitution of the 7(R)‑hydroxyl group of the parent antibiotic lincomycin.
The chemical name for clindamycin phosphate is Methyl 7-chloro-6,7,8-trideoxy-6- (1-methyl- trans-4-propyl-L-2-pyrrolidinecarboxamido)-1-thio-L-threo-α-D-galacto- octopyranoside 2-(dihydrogen phosphate). The structural formula for clindamycin phosphate is represented below:
Clindamycin phosphate:
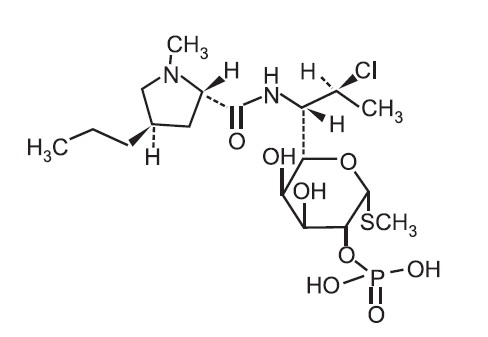
Molecular Formula: C18H34ClN2O8PS Molecular Weight: 504.97
Benzoyl peroxide is an antibacterial and keratolytic agent. The structural formula for benzoyl peroxide is represented below:
Benzoyl peroxide:
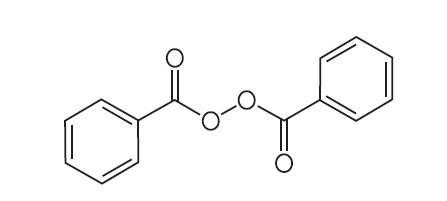
Molecular Formula: C14H10O4 Molecular Weight: 242.23
ONEXTON Gel contains the following inactive ingredients: carbomer 980, potassium hydroxide, propylene glycol, and purified water. Each gram of ONEXTON Gel contains 12 mg (1.2%) clindamycin phosphate, equivalent to 10 mg (1%) clindamycin, and 37.5 mg (3.75%) benzoyl peroxide.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Clindamycin: Clindamycin is a lincosamide antibacterial [see Microbiology (12.4)].
Benzoyl Peroxide: Benzoyl peroxide is an oxidizing agent with bactericidal and keratolytic effects but the precise mechanism of action is unknown.
12.3 Pharmacokinetics
The systemic absorption of ONEXTON Gel has not been evaluated. The systemic absorption of clindamycin was investigated in an open-label, multiple-dose trial in 16 adult subjects with moderate to severe acne vulgaris treated with 1 gram of a marketed gel containing clindamycin 1%/benzoyl peroxide 2.5% applied to the face once daily for 30 days. This product has the same formulation as ONEXTON Gel but with a lower concentration of benzoyl peroxide. Twelve subjects (75%) had at least one quantifiable clindamycin plasma concentration above the lower limit of quantification (LOQ = 0.5 ng/mL) on Day 1 or Day 30. On Day 1, the mean (± standard deviation) peak plasma concentrations (Cmax) was 0.78 ± 0.22 ng/mL (n=9 with measurable concentrations), and the mean AUC0-t was 5.29 ± 0.81 h.ng/mL (n=4). On Day 30, the mean Cmax was 1.22 ± 0.88 ng/mL (n=10), and the mean AUC0-t was 8.42 ± 6.01 h.ng/mL (n=6). Clindamycin plasma concentrations were below LOQ in all subjects at 24 hours post-dose on the three tested days (Day 1, 15, and 30).
Benzoyl peroxide has been shown to be absorbed by the skin where it is converted to benzoic acid.
12.4 Microbiology
Clindamycin binds to the 50S ribosomal subunits of susceptible bacteria and prevents elongation of peptide chains by interfering with peptidyl transfer, thereby suppressing bacterial protein synthesis.
Clindamycin and benzoyl peroxide individually have been shown to have in vitro activity against Propionibacterium acnes, an organism which has been associated with acne vulgaris. In an in vitro study, the minimum inhibitory concentration (MIC) for benzoyl peroxide against Propionibacterium acnes is 128 mg/L. The clinical significance of this activity against P. acnes is not known.
P. acnes resistance to clindamycin has been documented. Resistance to clindamycin is often associated with resistance to erythromycin.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
ONEXTON Gel, 1.2%/3.75% is a white to off-white smooth gel supplied as:
NDC 0187-3050-50 50 g pump
Dispensing Instructions for the Pharmacist
•
Dispense ONEXTON Gel with a 8-week expiration date.
•
Specify “Store at room temperature up to 25°C (77°F). Do not freeze.”
Storage and Handling
•
PHARMACIST: Prior to Dispensing: Store in a refrigerator, 2° to 8°C (36° to 46°F).
•
PATIENT: After Dispensing: Discard unused portion 8 weeks after date of dispensing. Store at room temperature at or below 25°C (77°F).
•
Protect from freezing.
•
Store pump upright.
•
Keep out of the reach of children
•
Keep container tightly closed.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
•
Patients who develop allergic reactions, such as severe swelling or shortness of breath, should discontinue use and contact their physician immediately.
•
ONEXTON Gel may cause irritation, such as erythema, scaling, itching, or burning, especially when used in combination with other topical acne therapies.
•
Patients should limit excessive or prolonged exposure to sunlight. To minimize exposure to sunlight, a hat or other clothing should be worn. Sunscreen may also be used.
•
ONEXTON Gel may bleach hair or colored fabric.
Distributed by:
Bausch Health US, LLC, Bridgewater, NJ 08807 USA
Manufactured by:
Bausch Health Companies Inc.
Laval, Quebec H7L 4A8, Canada
Patented. See https://patents.ortho-dermatologics.com for US patent information.
ONEXTON is a trademark of Bausch Health Companies Inc. or its affiliates.
© 2025 Bausch Health Companies Inc. or its affiliates
9432706
PATIENT MEDICATION INFORMATION SECTION
PATIENT INFORMATION
ONEXTON® (ON-EX-TUN)
(clindamycin phosphate and benzoyl peroxide) gel, 1.2%/3.75%
Important information: ONEXTON Gel is for use on skin only (topical use). Do not use ONEXTON Gel in your mouth, eyes, or vagina.
What is ONEXTON Gel?
ONEXTON Gel is a prescription medicine used on the skin (topical) to treat acne vulgaris in people 12 years of age and older.
It is not known if ONEXTON Gel is safe and effective for use longer than 12 weeks.
It is not known if ONEXTON Gel is safe and effective in children under 12 years of age.
Do not use ONEXTON Gel if you have:
•
had an allergic reaction to clindamycin, benzoyl peroxide, lincomycin, or any of the ingredients in ONEXTON Gel. See the end of this leaflet for a complete list of ingredients in ONEXTON Gel.
•
Crohn’s disease or ulcerative colitis.
•
had inflammation of the colon (colitis) or severe diarrhea with past antibiotic use.
Talk with your doctor if you are not sure if you have any of the conditions listed above.
Before using ONEXTON Gel, tell your doctor about all of your medical conditions, including if you:
•
plan to have surgery. ONEXTON Gel may affect how certain medicines work that may be given during surgery.
•
are pregnant or plan to become pregnant. It is not known if ONEXTON Gel will harm your unborn baby.
•
are breastfeeding or plan to breastfeed. It is not known if ONEXTON Gel passes into your breast milk. Clindamycin when taken by mouth or by injection has been reported to appear in breast milk. Talk to your doctor about the best way to feed your baby during treatment with ONEXTON Gel.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. ONEXTON Gel may affect the way other medicines work and other medicines may affect how ONEXTON Gel works.
•
Especially tell your doctor if you take medicine by mouth that contains erythromycin or use products on your skin that contain erythromycin. ONEXTON Gel should not be used with products that contain erythromycin.
•
Tell your doctor about any skin products you use. Other skin and topical acne products may increase the irritation of your skin when used with ONEXTON Gel.
Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine.
How should I use ONEXTON Gel?
•
Use ONEXTON Gel exactly as your doctor tells you to use it. See the detailed**“Instructions for Use”** for directions about how to apply ONEXTON Gel.
•
Your doctor will tell you how long to use ONEXTON Gel.
•
Apply ONEXTON Gel to your face 1 time each day.
Before you apply ONEXTON Gel, wash your face gently with a mild soap, rinse with warm water, and pat your skin dry.
What should I avoid while using ONEXTON Gel?
•
Limit your time in sunlight. You should avoid using tanning beds or sun lamps during treatment with ONEXTON Gel. If you have to be in sunlight, wear a wide-brimmed hat or other protective clothing, and use sunscreen to cover the treated areas.
•
Avoid getting ONEXTON Gel in your hair or on colored fabric. ONEXTON Gel may bleach hair or colored fabric.
What are possible side effects of ONEXTON Gel?
ONEXTON Gel can cause serious side effects including:
•
**Inflammation of the colon (colitis).** Stop using ONEXTON Gel and call your doctor right away if you have severe stomach (abdominal) cramps watery diarrhea, or bloody diarrhea during treatment, and within several weeks after treatment with ONEXTON Gel.
•
**Allergic reactions.** Stop using ONEXTON Gel, call your doctor and get help right away if you have any of the following symptoms during treatment with ONEXTON Gel:
o
severe itching
o
swelling of your face, eyes, lips, tongue or throat
o
trouble breathing
The most common side effects of ONEXTON Gel include burning sensation, skin redness or swelling, itching and rash. Stop using ONEXTON Gel and call your doctor if you have a skin rash or your skin becomes very red, itchy or swollen. Talk to your doctor about any side effect that bothers you or that does not go away.
These are not all of the possible side effects with ONEXTON Gel.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ONEXTON Gel?
•
Store ONEXTON Gel at room temperature at or below 77°F (25°C).
•
Do not freeze ONEXTON Gel.
•
Throw away (discard) ONEXTON Gel 8 weeks after date of dispensing.
•
Store pump upright.
•
Keep the container tightly closed.
Keep ONEXTON Gel and all medicines out of the reach of children.
General information about the safe and effective use of ONEXTON Gel.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ONEXTON Gel for a condition for which it was not prescribed. Do not give ONEXTON Gel to other people, even if they have the same condition you have. It may harm them. You can also ask your doctor or pharmacist for information about ONEXTON Gel that is written for healthcare professionals.
What are the ingredients in ONEXTON Gel?
Active Ingredients: clindamycin phosphate and benzoyl peroxide
Inactive Ingredients: carbomer 980, potassium hydroxide, propylene glycol, and purified water.
Manufactured by: Bausch Health Companies Inc., Laval, Quebec H7L 4A8, Canada
Distributed by: Bausch Health US, LLC, Bridgewater, NJ 08807 USA
Patented. See https://patents.ortho-dermatologics.com for US patent information.
ONEXTON is a trademark of Bausch Health Companies Inc. or its affiliates.
© 2025 Bausch Health Companies Inc. or its affiliates
For more information about ONEXTON Gel, call 1-800-321-4576.
This Patient Information has been approved by the U.S. Food and Drug
Administration. Revised: 07/2025
9432706
INSTRUCTIONS FOR USE SECTION
INSTRUCTIONS FOR USE
ONEXTON® (ON-EX-TUN)
(clindamycin phosphate and benzoyl peroxide) gel, 1.2%/3.75%
Important Information: ONEXTON Gel is for use on skin only (topical use). ONEXTON Gel is not for use in your mouth, eyes or vagina.
Read this Instructions for Use before you start using ONEXTON Gel and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or treatment.
•
Apply ONEXTON Gel to your face 1 time each day as prescribed.
•
Before you apply ONEXTON Gel, wash your face gently with a mild soap, rinse with warm water, and pat your skin dry.
•
To apply ONEXTON Gel to your face, use the pump to dispense one pea-sized amount of ONEXTON Gel onto your fingertip.**See Figure 1.**
•
One pea-sized amount of ONEXTON Gel should be enough to cover your entire face.
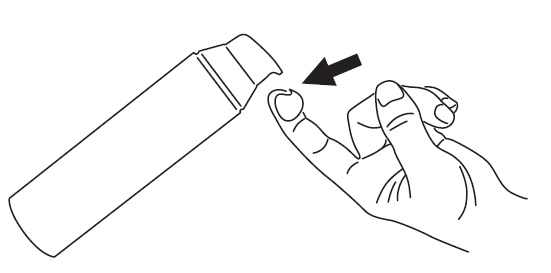
Figure 1
•
Dot the one pea-sized amount of ONEXTON Gel onto six areas of your face (chin, left cheek, right cheek, nose, left forehead, right forehead).**See Figure 2.**
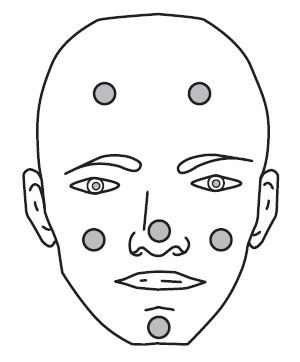
Figure 2
•
Spread the gel over your face and gently rub it in.**It is important to spread the gel over your entire face.** If your doctor tells you to put ONEXTON Gel on other areas of your skin with acne, be sure to ask how much you should use.
•
Wash your hands with soap and water after applying ONEXTON Gel.
How should I store ONEXTON Gel?
•
Store ONEXTON Gel at room temperature at or below 77°F (25°C).
•
Do not freeze ONEXTON Gel.
•
Throw away (discard) ONEXTON Gel 8 weeks after date of dispensing.
•
Store pump upright.
•
Keep the container tightly closed
Keep ONEXTON Gel and all medicines out of the reach of children.
Distributed by: Bausch Health US, LLC, Bridgewater, NJ 08807 USA
Manufactured by: Bausch Health Companies Inc., Laval, Quebec H7L 4A8,
Canada
Patented. See https://patents.ortho-dermatologics.com for US patent
information.
ONEXTON is a trademark of Bausch Health Companies Inc. or its affiliates.
© 2025 Bausch Health Companies Inc. or its affiliates
This Instructions for Use has been approved by the
U.S. Food and Drug Administration. Revised: July 2025
9432706
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Gel, 1.2%/3.75%
Each gram of ONEXTON Gel contains 12 mg (1.2%) clindamycin phosphate, equivalent to 10 mg (1%) clindamycin, and 37.5 mg (3.75%) benzoyl peroxide in a white to off-white, opaque, smooth gel.
Gel, 1.2% clindamycin phosphate/3.75% benzoyl peroxide (3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on ONEXTON Gel use in pregnant women to evaluate a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. The limited published data on use of clindamycin in pregnant women with exposure during the first trimester are insufficient to inform a drug-associated risk of pregnancy-related adverse outcomes (see Data). In limited published clinical trials with pregnant women, the systemic administration of clindamycin during the second and third trimesters has not been associated with an increased frequency of major birth defects.
In animal reproduction studies, clindamycin did not cause malformations or embryo-fetal development toxicity in pregnant rats and mice when administered during the period of organogenesis at systemic doses up to 240 times the maximum recommended human dose (MRHD) of 2.5 g ONEXTON Gel, based on body surface area (BSA) comparisons (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of major birth defects, loss, and other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Human Data
In limited published trials in pregnant women administered clindamycin during the first trimester of pregnancy, there was no difference in the rate of major birth defects reported among in utero exposed infants compared to unexposed infants. These data cannot definitely establish or exclude any clindamycin- associated risk during pregnancy.
Animal Data
Animal reproductive/developmental toxicity studies have not been conducted with ONEXTON Gel or benzoyl peroxide. Developmental toxicity studies of clindamycin performed in pregnant rats and mice administered during the period of organogenesis at oral doses of up to 600 mg/kg/day (240 and 120 times the MRHD for clindamycin, respectively, based on BSA comparisons) or subcutaneous doses of up to 200 mg/kg/day (80 and 40 times the MRHD for clindamycin, respectively, based on BSA comparisons) revealed no malformations or embryo- fetal development toxicity.
8.2 Lactation
Risk Summary
There are no data on the presence of clindamycin or benzoyl peroxide in human milk, the effects on the breastfed child, or the effects on milk production following topical administration. However, clindamycin has been reported to be present in breast milk in small amounts following oral and parenteral administration. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ONEXTON Gel and any potential adverse effects on the breastfed child from ONEXTON Gel or from the underlying maternal condition.
Clinical Considerations
If used during lactation and ONEXTON Gel is applied to the chest, care should be taken to avoid accidental ingestion by the infant.
8.4 Pediatric Use
Safety and effectiveness of ONEXTON Gel in pediatric patients under the age of 12 years have not been evaluated.
8.5 Geriatric Use
Clinical trials of ONEXTON Gel did not include sufficient numbers of subjects age 65 years and older to determine whether they respond differently from younger subjects.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity, mutagenicity, and impairment of fertility testing of ONEXTON Gel have not been performed.
Benzoyl peroxide has been shown to be a tumor promoter and progression agent in a number of animal studies. Benzoyl peroxide in acetone at doses of 5 and 10 mg administered topically twice per week for 20 weeks induced skin tumors in transgenic Tg.AC mice. The clinical significance of this is unknown.
Carcinogenicity studies have been conducted with a gel formulation containing 1% clindamycin and 5% benzoyl peroxide. In a 2-year dermal carcinogenicity study in mice, treatment with the gel formulation at doses of 900, 2700, and 15000 mg/kg/day (1.8, 5.4, and 30 times the MRHD for clindamycin and 2.4, 7.2, and 40 times the MRHD for benzoyl peroxide, respectively, based on BSA comparisons) did not cause any increase in tumors. However, topical treatment with a different gel formulation containing 1% clindamycin and 5% benzoyl peroxide at doses of 100, 500, and 2000 mg/kg/day caused a dose-dependent increase in the incidence of keratoacanthoma at the treated skin site of male rats in a 2-year dermal carcinogenicity study in rats. In an oral (gavage) carcinogenicity study in rats, treatment with the gel formulation at doses of 300, 900, and 3000 mg/kg/day (1.2, 3.6, and 12 times the MRHD for clindamycin and 1.6, 4.8, and 16 times the MRHD for benzoyl peroxide, respectively, based on BSA comparisons) for up to 97 weeks did not cause any increase in tumors.
Clindamycin phosphate was not genotoxic in the human lymphocyte chromosome aberration assay. Benzoyl peroxide has been found to cause DNA strand breaks in a variety of mammalian cell types, to be mutagenic in S. typhimurium tests by some but not all investigators, and to cause sister chromatid exchanges in Chinese hamster ovary cells.
Fertility studies have not been performed with ONEXTON Gel or benzoyl peroxide, but fertility and mating ability have been studied with clindamycin. Fertility studies in rats treated orally with up to 300 mg/kg/day of clindamycin (approximately 120 times the MRHD for clindamycin based on BSA comparisons) revealed no effects on fertility or mating ability.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
The safety and efficacy of once-daily use of ONEXTON Gel was assessed in a 12-week multi-center, randomized, blinded trial in subjects 12 years and older with moderate to severe acne vulgaris. This trial evaluated ONEXTON Gel compared to vehicle gel.
The co-primary efficacy variables for this trial were:
(1) Mean absolute change from baseline at Week 12 in:
•
Inflammatory lesion counts
•
Non-inflammatory lesion counts
(2) Percent of subjects who had a 2-grade reduction from baseline on an Evaluator’s Global Severity (EGS) score.
The EGS scoring scale used in the clinical trial for ONEXTON Gel is as follows:
Table 2: EGS Scoring Scale
|
Grade |
Description |
|---|---|
|
Clear |
Normal, clear skin with no evidence of acne |
|
Almost Clear |
Rare non-inflammatory lesions present, with rare non-inflamed papules (papules must be resolving and may be hyperpigmented, though not pink-red) |
|
Mild |
Some non-inflammatory lesions are present, with few inflammatory lesions (papules/pustules only; no nodulocystic lesions) |
|
Moderate |
Non-inflammatory lesions predominate, with multiple inflammatory lesions evident: several to many comedones and papules/pustules, and there may or may not be one small nodulocystic lesion |
|
Severe |
Inflammatory lesions are more apparent, many comedones and papules/pustules, there may or may not be up to 2 nodulocystic lesions |
|
Very Severe |
Highly inflammatory lesions predominate, variable number of comedones, many papules/pustules and more than 2 nodulocystic lesions |
The results of the trial at Week 12 are presented in Table 3:
Table 3: Results of Phase 3 Trial at Week 12
|
ONEXTON Gel |
Vehicle Gel | |
|---|---|---|
|
EGSS: |
29% |
15% |
|
35% |
17% |
|
Inflammatory Lesions: | ||
|
16.3 |
8.2 |
|
60.4% |
31.3% |
|
Non-Inflammatory Lesions: | ||
|
19.2 |
9.6 |
|
51.8% |
27.6% |
