Bee Venom Toenail Fungus Treatment
3e7d22b0-2f57-fcac-e063-6394a90aefd9
HUMAN OTC DRUG LABEL
Sep 10, 2025
Shenzhen Earth Surface Trading Co., Ltd.
DUNS: 454290055
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Bee Venom Toenail Fungus Treatment
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
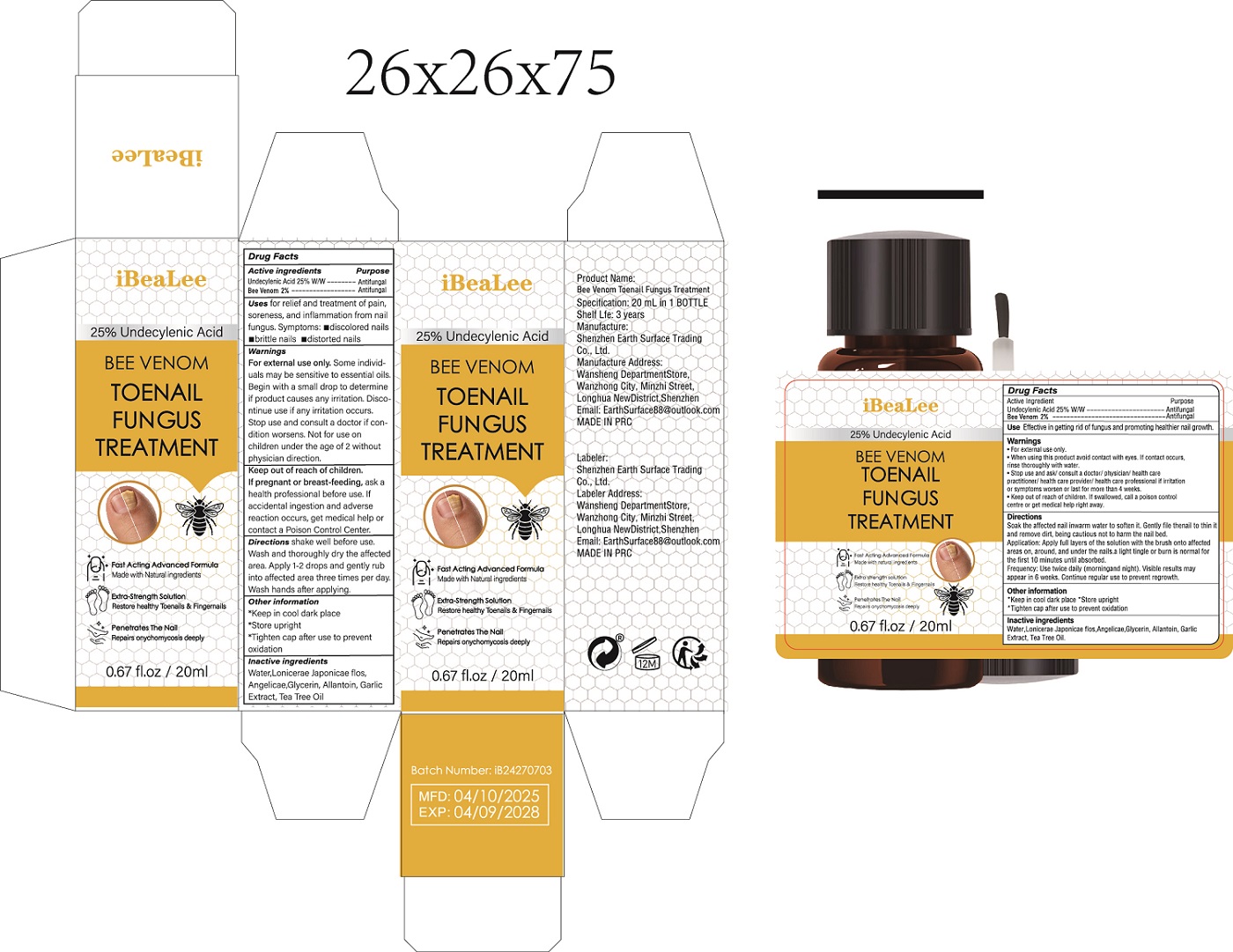
INDICATIONS & USAGE SECTION
Uses
Effective in getting rid of fungus and promoting healthier nail growth.
INACTIVE INGREDIENT SECTION
inactive ingredients
Water
OTC - ACTIVE INGREDIENT SECTION
Active ingredients
Undecylenic Acid 25% W/W
Bee Venom 2%
OTC - PURPOSE SECTION
PURPOSE
Antifungal
WARNINGS SECTION
Warnings
• For external use only.
• When using this product avoid contact with eyes. If contact occurs,
rinse thoroughly with water.
• Stop use and ask/ consult a doctor/ physician/ health care
practitioner/ health care provider/ health care professional if irritation
or symptoms worsen or last for more than 4 weeks.
• Keep out of reach of children. If swallowed, call a poison control
centre or get medical help right away、
OTC - STOP USE SECTION
Stop use and ask a doctor if
if irritation or symptoms worsen
or last for more than 4 weeks.
OTC - DO NOT USE SECTION
Do not use
• If you are pregnant or breastfeeding
• your skin is injured or cracked.
• you are under 12 years of age.
• irritation or discomfort occurs.
• you are using other medicines on your nails.
OTC - WHEN USING SECTION
When using this product
• Do not apply to other parts of the body.
• Avoid making eye contact. In case of accidental eye contact, flush
eyes with copious amounts of cold tap water.
• This product may not work for everyone.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children.
If swallowed, get medical help or contact a poison control center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
Soak the affected nail inwarm water
to soften it. Gently file the nail to
thin it and remove dirt, being cau-
tious not to harm the nail bed.
Application: Apply full layers of
the solution with the brush onto
affected areas on, around, and
under the nails.a light tingle or burn
is normal for the first 10 minutes
until absorbed.
Frequency: Use twice daily
(morningand night). Visible results
may appear in 6 weeks. Continue
regular use to prevent regrowth.
