Tropicamide
Tropicamide Ophthalmic Solution, USP 1%Somerset Therapeutics, LLC
378b5fe2-4b5d-4c87-a7d6-fd0caa6402af
HUMAN PRESCRIPTION DRUG LABEL
Apr 26, 2023
Somerset Therapeutics, LLC
DUNS: 079947873
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Tropicamide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
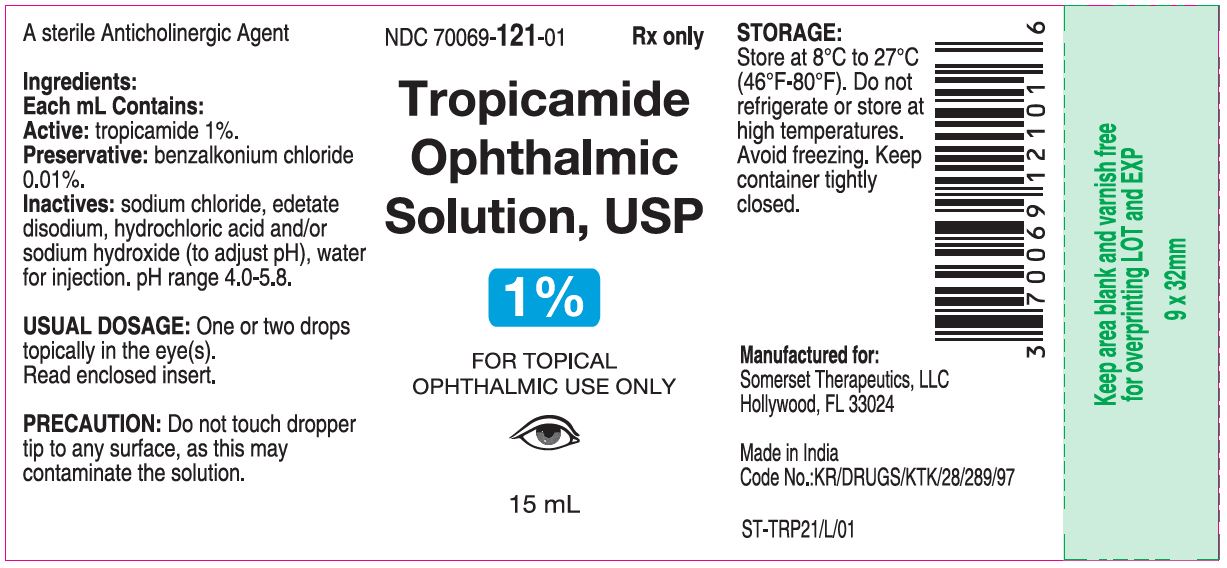
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
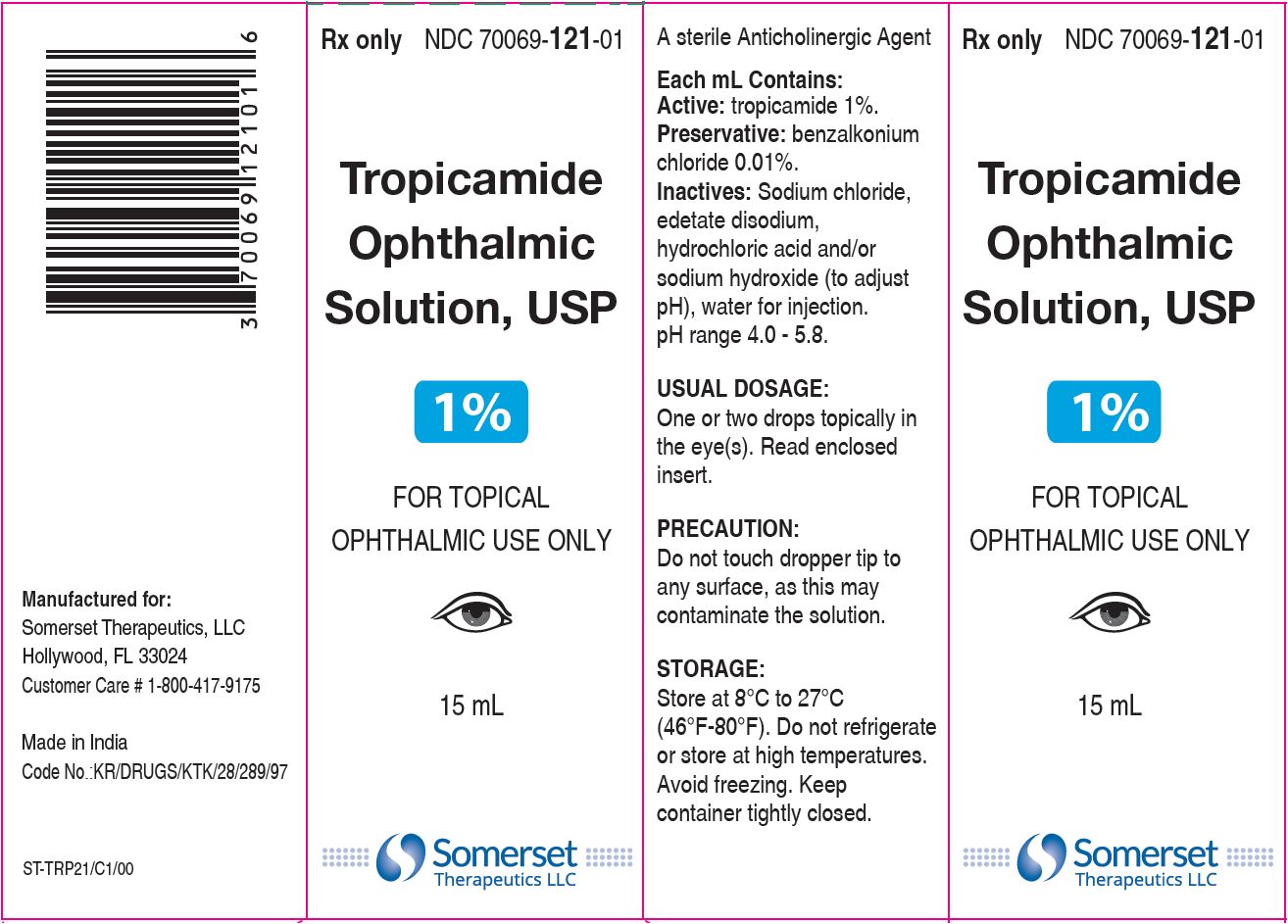
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Container Label
Carton Label
DESCRIPTION SECTION
DESCRIPTION
Tropicamide Ophthalmic Solution, USP is an anticholinergic prepared as a sterile topical ophthalmic solution. The active ingredient is represented by the chemical structure:
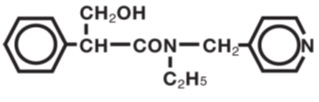
Established name:
Tropicamide ophthalmic solution, USP
Chemical name:
Benzeneacetamide, N-ethyl-α-(hydroxymethyl)-N-(4-pyridinylmethyl).
**Each mL contains: Active:tropicamide 1%. Preservative:benzalkonium chloride 0.01%. Inactives:**sodium chloride, edetate disodium, hydrochloric acid and/or sodium hydroxide (to adjust pH), Water for injection. pH range 4.0
- 5.8.
PRECAUTIONS SECTION
PRECAUTIONS
General
The lacrimal sac should be compressed by digital pressure for two to three minutes after instillation to reduce excessive systemic absorption.
Information for Patients
Do not touch dropper tip to any surface, as this may contaminate the solution. Patient should be advised not to drive or engage in potentially hazardous activities while pupils are dilated. Patient may experience sensitivity to light and should protect eyes in bright illumination during dilation. Parents should be warned not to get this preparation in their child's mouth and to wash their own hands and the child's hands following administration.
Drug Interactions
Tropicamide may interfere with the antihypertensive action of carbachol, pilocarpine, or ophthalmic cholinesterase inhibitors.
Carcinogenesis, Mutagenesis, Impairment of Fertility
There have been no long-term studies done using tropicamide in animals to evaluate carcinogenic potential.
Pregnancy
Animal reproduction studies have not been conducted with tropicamide. It is also not known whether tropicamide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Tropicamide should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when tropicamide is administered to a nursing woman.
Pediatric Use
Tropicamide may rarely cause CNS disturbances which may be dangerous in pediatric patients. Psychotic reactions, behavioral disturbances, and vasomotor or cardiorespiratory collapse in children have been reported with the use of anticholinergic drugs (See WARNINGS). Keep this and all medications out of the reach of children.
Geriatric Use
No overall differences in safety or effectiveness have been observed between elderly and younger patients.
