Magnesium Sulfate
d4b5863a-efb9-4551-a9ad-2b06e8666d87
HUMAN PRESCRIPTION DRUG LABEL
Jul 17, 2023
Hospira, Inc.
DUNS: 141588017
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
magnesium sulfate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
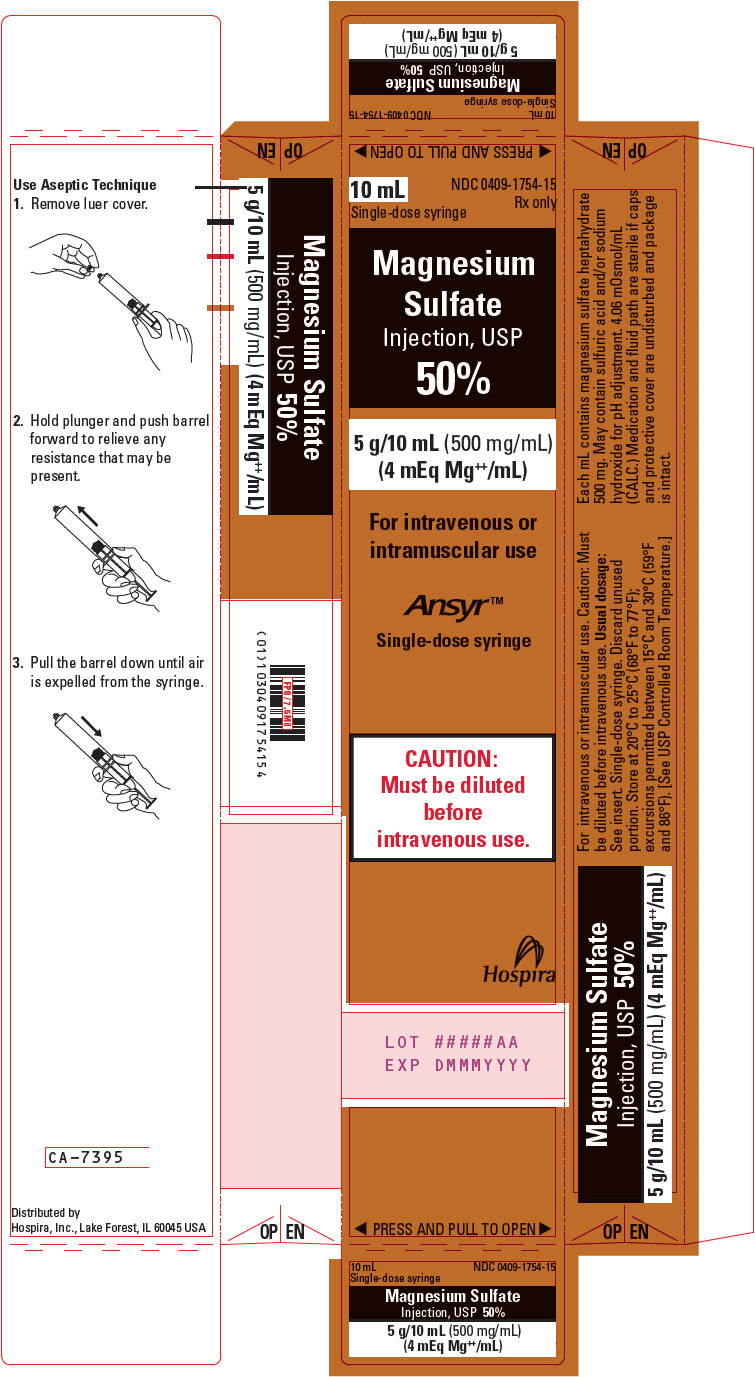
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 10 mL Syringe Carton
10 mL
NDC 0409-1754-15
Rx only
Single-dose syringe
Magnesium
Sulfate
Injection, USP
50%
5 g/10 mL (500 mg/mL)
(4 mEq Mg++/mL)
For intravenous or
intramuscular use
Ansyr™
Single-dose syringe
CAUTION:
Must be diluted
before
intravenous use.
Hospira
LOT #####AA
EXP DMMMYYYY
◀ PRESS AND PULL TO OPEN ▶
HOW SUPPLIED SECTION
HOW SUPPLIED
Magnesium Sulfate Injection, USP is supplied in single-dose containers as follows:
|
NDC No. |
Container |
Total Amount |
Concentration |
mEq Mg**++****/mL** |
|
0409-1754-10 |
Ansyr™ Plastic Syringe |
5 g/10 mL |
50% |
4 mEq/mL |
Do not administer unless solution is clear and container is undamaged. Discard unused portion.
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F). [See USP Controlled Room Temperature.]
REFERENCES SECTION
REFERENCES
Yokoyama K, Takahashi N, Yada Y. Prolonged maternal magnesium administration and bone metabolism in neonates. Early Hum Dev. 2010;86(3):187-91. Epub 2010 Mar 12.
Wedig KE, Kogan J, Schorry EK et al. Skeletal demineralization and fractures caused by fetal magnesium toxicity. J. Perinatol. 2006; 26(6):371-4.
Nassar AH, Sakhel K, Maarouf H, et al. Adverse maternal and neonatal outcome of prolonged course of magnesium sulfate tocolysis. Acta Obstet Gynecol Scan. 2006;85(9):1099-103.
Malaeb SN, Rassi A, Haddad MC. Bone mineralization in newborns whose mothers received magnesium sulphate for tocolysis of premature labor. Pediatr Radiol. 2004;34(5):384-6. Epub 2004 Feb 18.
Matsuda Y, Maeda Y, Ito M, et al. Effect of magnesium sulfate treatment on neonatal bone abnormalities. Gynecol Obstet Invest. 1997;44(2):82-8.
Schanler RJ, Smith LG, Burns PA. Effects of long-term maternal intravenous magnesium sulfate therapy on neonatal calcium metabolism and bone mineral content. Gynecol Obstet Invest. 1997;43(4):236-41.
Santi MD, Henry GW, Douglas GL. Magnesium sulfate treatment of preterm labor as a cause of abnormal neonatal bone mineralization. J Pediatr Orthrop. 1994;14(2):249-53.
Holcomb WL, Shackelford GD, Petrie RH. Magnesium tocolysis and neonatal bone abnormalities; a controlled study. Obstet Gynecol. 1991; 78(4):611-4.
Cumming WA, Thomas VJ. Hypermagnesemia: a cause of abnormal metaphyses in the neonate. Am J Roentgenol. 1989; 152(5):1071-2.
Lamm CL, Norton KL, Murphy RJ. Congenital rickets associated with magnesium sulfate infusion for tocolysis. J Pediatr. 1988; 113(6):1078-82.
McGuinness GA, Weinstein MM, Cruikshank DP, et al. Effects of magnesium sulfate treatment on perinatal calcium metabolism. II. Neonatal responses. Obstet Gynecol. 1980; 56(5): 595-600.
Riaz M, Porat R, Brodsky NL, et al. The effects of maternal magnesium sulfate treatment on newborns: a prospective controlled study. J. Perinatol. 1998;18(6 pt 1):449-54.
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA 
LAB-1024-4.0
Revised: 05/2020
