Perphenazine
Perphenazine Tablets, USP
9ad131c1-0620-4d3c-9734-bcffd98430e2
HUMAN PRESCRIPTION DRUG LABEL
Sep 12, 2023
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
perphenazine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
BOXED WARNING SECTION
INDICATIONS & USAGE SECTION
INDICATIONS AND USAGE
Perphenazine is indicated for use in the treatment of schizophrenia and for the control of severe nausea and vomiting in adults.
Perphenazine has not been shown effective for the management of behavioral complications in patients with mental retardation.
SPL UNCLASSIFIED SECTION
Dist. by:
Par Pharmaceutical
Chestnut Ridge, NY 10977 U.S.A.
Mfg. by:
Par Formulations Private Limited,
9/215, Pudupakkam, Kelambakkam - 603 103.
Made in India
Mfg. Lic. No.: TN00002121
OS5060-01-74-01
Issued: 10/2020
MECHANISM OF ACTION SECTION
ACTIONS
Perphenazine has actions at all levels of the central nervous system, particularly the hypothalamus. However, the site and mechanism of action of therapeutic effect are not known.
DESCRIPTION SECTION
DESCRIPTION
Perphenazine (4-[3-(2-chlorophenothiazin-10-yl)propyl]-1-piperazineethanol), a piperazinyl phenothiazine, having the chemical formula, C21H26CIN3OS. It is available as oral tablets containing 2 mg, 4 mg, 8 mg, and 16 mg of perphenazine.
Inactive ingredients: black iron oxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, sodium starch glycolate, talc, titanium dioxide, yellow iron oxide. Its structural formula is:
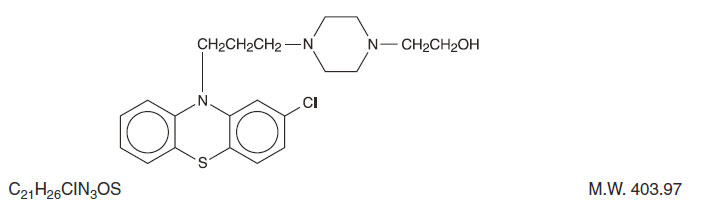
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
Pharmacokinetics
Following oral administration of perphenazine tablets, mean peak plasma perphenazine concentrations were observed between 1 to 3 hours. The plasma elimination half-life of perphenazine was independent of dose and ranged between 9 and 12 hours. In a study in which normal volunteers (n=12) received perphenazine 4 mg q8h for 5 days, steady-state concentrations of perphenazine were reached within 72 hours. Mean (%CV) Cmax and Cmin values for perphenazine and 7-hydroxyperphenazine at steady-state are listed below:
|
Parameter |
Perphenazine |
7-Hydroxyperphenazine |
|
Cmax (pg/mL) |
984 (43) |
509 (25) |
|
Cmin (pg/mL) |
442 (76) |
350 (56) |
Peak 7-hydroxyperphenazine concentrations were observed between 2 to 4 hours with a terminal phase half-life ranging between 9.9 to 18.8 hours. Perphenazine is extensively metabolized in the liver to a number of metabolites by sulfoxidation, hydroxylation, dealkylation, and glucuronidation. The pharmacokinetics of perphenazine covary with the hydroxylation of debrisoquine which is mediated by cytochrome P450 2D6 (CYP 2D6) and thus is subject to genetic polymorphism—i.e., 7% to 10% of Caucasians and a low percentage of Asians have little or no activity and are called “poor metabolizers.” Poor metabolizers of CYP 2D6 will metabolize perphenazine more slowly and will experience higher concentrations compared with normal or “extensive” metabolizers.
