TopCare Omeprazole delayed release
Topco Associates LLC. Omeprazole Delayed Release Tablets Drug Facts
31194780-c051-6d98-e063-6294a90a9a54
HUMAN OTC DRUG LABEL
Sep 22, 2025
Sixarp, LLC
DUNS: 016329513
Praxis, LLC
DUNS: 016329513
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Omeprazole
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (20)
Drug Labeling Information
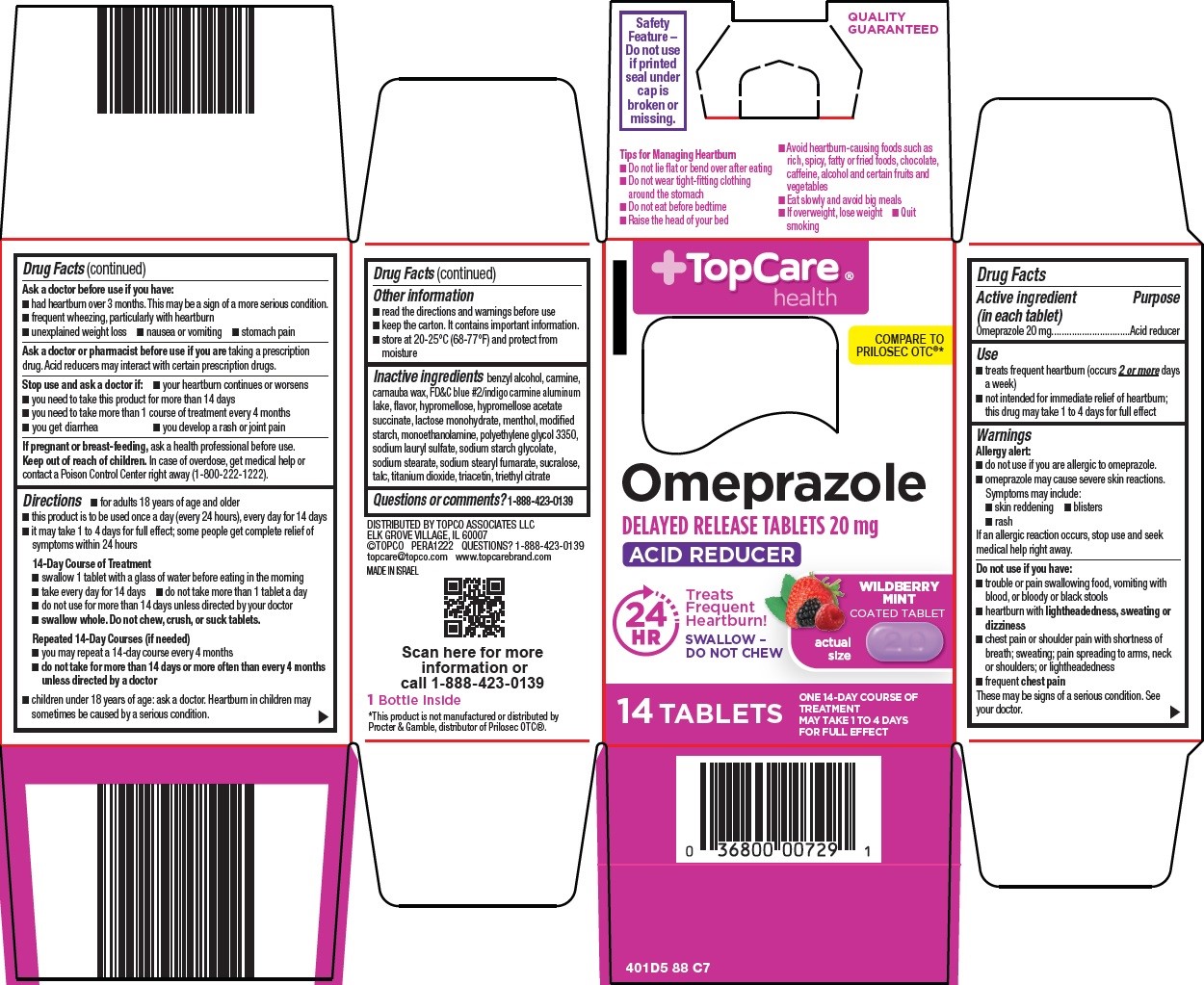
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Principal Display Panel
TopCare ®health
COMPARE TO PRILOSEC OTC ®
Omeprazole
DELAYED RELEASE TABLETS 20 mg
ACID REDUCER
24 HR
Treats Frequent Heartburn!
WILDBERRY MINT COATED TABLET
SWALLOW – DO NOT CHEW
actual size
14 TABLETS
ONE 14-DAY COURSE OF TREATMENT
MAY TAKE 1 TO 4 DAYS FOR FULL EFFECT