Acamprosate Calcium
These highlights do not include all the information needed to use ACAMPROSATE CALCIUM DELAYED-RELEASE TABLETS safely and effectively. See full prescribing information for ACAMPROSATE CALCIUM DELAYED-RELEASE TABLETS. ACAMPROSATE CALCIUM delayed-release tablets, for oral use Initial U.S. Approval: 2004
94ef2a5c-cc2f-4e7f-b159-b2d38188d5b8
HUMAN PRESCRIPTION DRUG LABEL
Aug 26, 2025
American Health Packaging
DUNS: 929561009
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Acamprosate Calcium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Display Panel – Blister – 333 mg

Acamprosate
Calcium
Delayed-Release
Tablet
333 mg
DESCRIPTION SECTION
11 DESCRIPTION
Acamprosate calcium delayed-release tablets are supplied as an enteric-coated tablet for oral administration. Acamprosate calcium, USP is a synthetic compound with a chemical structure similar to that of the endogenous amino acid homotaurine, which is a structural analogue of the amino acid neurotransmitter γ-aminobutyric acid and the amino acid neuromodulator taurine. Its chemical name is calcium bis [3-(acetylamino) propane-1-sulfonate]. Its chemical formula is C 10H 20CaN 2O 8S 2 and molecular weight is 400.5 g/mol. Its structural formula is:
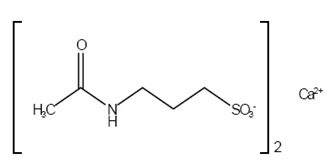
Acamprosate calcium, USP is a white to almost white powder, odorless or nearly odorless powder. It is freely soluble in water and practically insoluble in alcohol and methylene chloride.
Each acamprosate calcium delayed-release tablet contains acamprosate calcium USP, 333 mg, equivalent to 300 mg of acamprosate. Inactive ingredients in acamprosate calcium delayed-release tablets include: colloidal silicon dioxide, crospovidone, methacrylic acid copolymer dispersion, magnesium silicate, magnesium stearate, microcrystalline cellulose, polyvinyl pyrollidone, propylene glycol, sodium starch glycolate, and talc. Sulfites were used in the synthesis of the drug substance and traces of residual sulfites may be present in the drug product.
