Propranolol Hydrochloride
Propranolol Hydrochloride Extended-Release Capsules, USP 8421501/0725F Rx Only
015b5e4f-3228-4259-b419-e0e49694a058
HUMAN PRESCRIPTION DRUG LABEL
Sep 19, 2025
American Health Packaging
DUNS: 929561009
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Propranolol Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Propranolol Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (14)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Display Panel – Blister – 80 mg
Propranolol
Hydrochloride
Extended-Release
Capsule, USP
80 mg
SPL UNCLASSIFIED SECTION
PACKAGING INFORMATION
American Health Packaging unit dose blisters (see How Supplied section)
contain drug product from Breckenridge Pharmaceutical, Inc. as follows:
(60 mg / 100 UD) NDC 60687-215-01 packaged from NDC 51991-817
(80 mg / 100 UD) NDC 60687-226-01 packaged from NDC 51991-818
Distributed by:
American Health Packaging
Columbus, OH 43217
8421501/0725F
DESCRIPTION SECTION
DESCRIPTION
Propranolol hydrochloride is a synthetic beta-adrenergic receptor-blocking agent chemically described as 2-Propanol, 1-[(1-methylethyl)amino]-3-(1-naphthalenyloxy)-, hydrochloride,(±)-. Its molecular and structural formulae are:
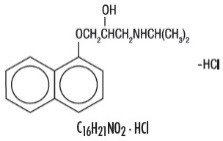
C 16H 21NO 2 · HCl
Propranolol hydrochloride is a stable, white, crystalline solid which is readily soluble in water and ethanol. Its molecular weight is 295.80.
Propranolol Hydrochloride Extended-Release Capsules, USP, are formulated to provide a sustained release of propranolol hydrochloride.
Propranolol Hydrochloride Extended-Release Capsules, USP, are available as 60 mg, 80 mg, 120 mg, and 160 mg capsules for oral administration.
Each capsule for oral administration contains sugar spheres, ethylcellulose, hypromellose phthalate, povidone, diethyl phthalate, polyethylene glycol, titanium dioxide, ammonium hydroxide, potassium hydroxide, black iron oxide, and gelatin. The 80 mg, 120 mg, and 160 mg capsules contain red and yellow iron oxide. In addition, the 160 mg capsules contain FD&C Blue No. 2.
These capsules comply with USP Dissolution Test 1.
HOW SUPPLIED SECTION
HOW SUPPLIED
Propranolol Hydrochloride Extended-Release Capsules, USP.
Each white/opaque capsule, imprinted with "60"on cap and "RD203" on body contains 60 mg of propranolol hydrochloride in unit dose packages of 100 (10 x 10) NDC 60687-215-01.
Each capsule with white/opaque body and orange/opaque cap, imprinted with "80"on cap and "RD203" on body contains 80 mg of propranolol hydrochloride in unit dose packages of 100 (10 x 10) NDC 60687-226-01.
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Protect from light, moisture, freezing, and excessive heat.
**FOR YOUR PROTECTION:**Do not use if blister is torn or broken.
Propranolol Hydrochloride Extended-Release Capsules, USP, a DIFFUCAPS ® drug delivery product, manufactured by Adare Pharmaceuticals, Inc.
You can also ask your doctor or pharmacist for information about Propranolol Hydrochloride Extended-Release Capsules, USP that is written for healthcare professionals. For more information about the drug product, call 1-800-367-3395 or go to www.breckenridgepharma.com.
For more information about the packaging or labeling, call American Health Packaging at 1‐800‐707‐4621.
