Xpect Stopain Clinical
Xpect Stopain Clinical Maximum Performance Pain Relief
e911478f-7153-4700-b945-92ec54972f45
HUMAN OTC DRUG LABEL
May 21, 2025
Cintas Corporation
DUNS: 056481716
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Menthol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Dispay 42961-257-01
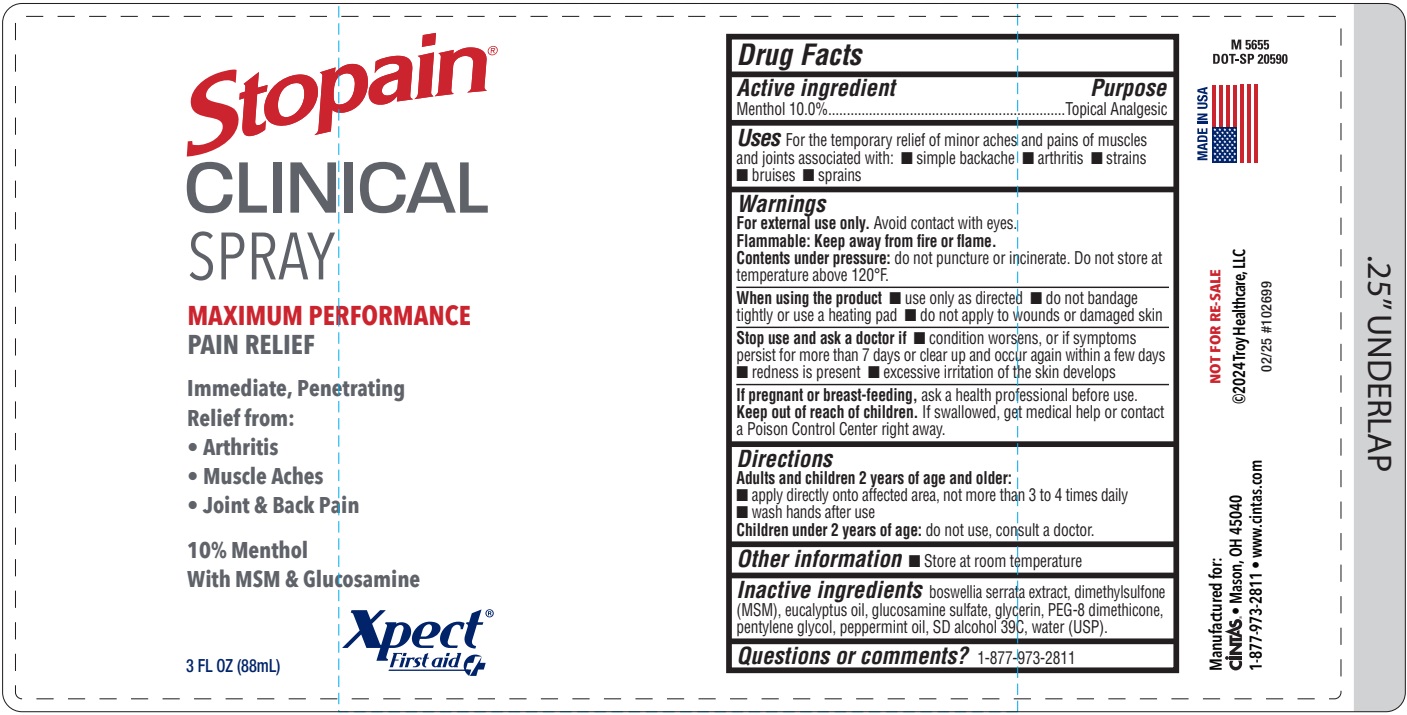
Xpect Stopain Clinical Spray Label
INDICATIONS & USAGE SECTION
Uses
For the temporary relief of minor aches and pains of muscles and joints associated with:
- simple backache
- arthritis
- strains
- bruises
- sprains
INACTIVE INGREDIENT SECTION
Inactive ingredients
boswellia serrata extract, dimethylsulfone (MSM), eucalyptus oil, glucosamine sulfate, glycerin, PEG-8 dimethicone, pentylene glycol, peppermint oil, SD alcohol 39C, water (USP).
OTC - QUESTIONS SECTION
Questions or comments?
1-877-973-2811
SPL UNCLASSIFIED SECTION
OTC - ACTIVE INGREDIENT SECTION
Active ingredient
Menthol 10.0%
OTC - PURPOSE SECTION
Purpose
Topical Analgesic
WARNINGS SECTION
Warnings
For external use only. Avoid contact with eyes.
Flammable: Keep away from fire or flame.
Contents under pressure: do not puncture or incinerate. Do not store at temperature above 120°F.
OTC - WHEN USING SECTION
When using the product
- use only as directed
- do not bandage tightly or use a heating pad
- do not apply to wounds or damaged skin
OTC - STOP USE SECTION
Stop use and ask a doctor if
- condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days
- redness is present
- excessive irritation of the skin develops
OTC - PREGNANCY OR BREAST FEEDING SECTION
If pregnant or breast-feeding, ask a health professional before use.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
Adults and children 2 years of age and older:
- apply directly onto affected area, not more than 3 to 4 times daily
- wash hands after use
Children under 2 years of age: do not use, consult a doctor
OTHER SAFETY INFORMATION
Other information
- Store at room temperature
