MAGNESIUM SULFATE IN DEXTROSE
These highlights do not include all the information needed to use MAGNESIUM SULFATE IN 5% DEXTROSE INJECTION safely and effectively. See full prescribing information for MAGNESIUM SULFATE IN 5% DEXTROSE INJECTION.MAGNESIUM SULFATE IN DEXTROSE injection, for intravenous useInitial U.S. Approval: 1941
1bbaebd0-3f4c-467d-96fd-69cfad5a3fd3
HUMAN PRESCRIPTION DRUG LABEL
Nov 30, 2020
Baxter Healthcare Corporation
DUNS: 005083209
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
magnesium sulfate in dextrose
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Made in Spain

Container Label
100 mL
NDC 0338-1709-40
Magnesium Sulfate
in5% Dextrose Injection, USP
1 g/ 100 mL (10 mg/mL)
1 g
TOTAL
Each 100 mL of sterile, nonpyrogenic solution contains:
Magnesium Sulfate Heptahydrate** 1** g (equivalent to8.1 mEq
magnesium) and dextrose, hydrous5 g in water for injection. May
contain sulfuric acid and/or sodium hydroxide for pH adjustment.
pH4.5 (3.5 to6.5)333mOsmol/Liter (calc.)
Single-Dose Container – Discard unused portion. For Intravenous Use.
Recommended dosage: See prescribing information. Use only if
solution is clear and container is undamaged. Must not be used
in series connections. Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.] Protect from freezing.
VIAFLO container is not made with natural rubber latex, DEHP, or PVC.
Rx Only
Number 07 plastics symbol 0
Bar code
(01) 00303381709402
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in Spain
UE8501
UN-35-04-602
DO NOT USE
THIS PORT symbol
Lot
Exp

Overpouch Label
100 mL
TO OPEN – TEAR AT NOTCH
NDC 0338-1709-40
Magnesium Sulfate
in 5% Dextrose Injection, USP
1 g/100 mL (10 mg/mL)
1g
Total
Each 100 mL of sterile, nonpyrogenic solution contains:
Magnesium Sulfate Heptahydrate 1 g (equivalent to 8.1 mEq magnesium) and
dextrose, hydrous 5 g in water for injection. May contain sulfuric acid and/or
sodium hydroxide for pH adjustment.
333 mOsmol/Liter (calc.) pH 4.5 (3.5 to 6.5)
DO NOT ADD SUPPLEMENTARY MEDICATION. WHENEVER POSSIBLE USE
CENTRAL ROUTE.
Single-Dose Container – Discard unused portion. For Intravenous Use.
Recommended dosage: See prescribing information. Use only if solution is
clear and container is undamaged. After removing the overwrap, check for
minute leaks by squeezing container firmly. If leaks are found, discard unit
as
sterility may be impaired. Must not be used in series connections.
The overwrap is a moisture barrier. Do not remove unit from overwrap until
ready for use. Use unit promptly when pouch is opened.
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
Protect from freezing. VIAFLO container is not made with natural rubber latex,
DEHP, or PVC.
2D Barcode
(91)SA1001003
Rx Only
UN8501
SA-10-01-003
Baxter Logo
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in Spain
(See Solution Container for Lot and Exp)
Barcode
(01) 00303381709402
DESCRIPTION SECTION
11 DESCRIPTION
Magnesium Sulfate in 5% Dextrose Injection, USP is a sterile, nonpyrogenic solution of magnesium sulfate heptahydrate and dextrose in water for injection for intravenous use. Each 100 mL contains 1 gram of magnesium sulfate heptahydrate and dextrose, hydrous 5 grams in water for injection [see How Supplied/Storage and Handling (16)]. Magnesium Sulfate in 5% Dextrose Injection, USP may contain sulfuric acid and/or sodium hydroxide for pH adjustment. The pH is 4.5 (3.5 to 6.5).
Magnesium Sulfate, USP heptahydrate is chemically known as sulfuric acid magnesium salt (1:1), heptahydrate and chemically designated MgSO4 • 7H2O, with a molecular weight of 246.47. It occurs as colorless crystals or white powder freely soluble in water.
Dextrose, USP is chemically designated D-glucose, monohydrate, a hexose sugar freely soluble in water. The molecular formula is C6H12O6 • H2O and the molecular weight is 198.17. It has the following structural formula:
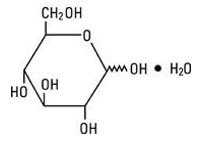
Water for Injection, USP is chemically designated H2O.
Water can permeate from inside the Baxter VIAFLO flexible plastic container [composed of Polypropylene (PP), Polyamide (PA) and Polyethylene (PE)] into the overwrap [see Dosage and Administration (2.1)] but not in amounts sufficient to affect the solution significantly. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials. Exposure to temperatures above 25°C (77°F) during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Magnesium Sulfate in 5% Dextrose Injection, USP is a clear solution supplied in VIAFLO single-dose flexible plastic containers (see Table 2).
Table 2 : How Supplied Information|
Magnesium Sulfate Concentration* |
NDC Number |
Container |
Total Magnesium |
Total Magnesium Ion |
Magnesium Ion Concentration |
Osmolarity** |
|
0.01 grams/mL (1%) |
0338-1709-40 |
100 mL |
1 gram |
8.1 mEq |
8.1 mEq/100 mL |
333 mOsmol/liter |
VIAFLO container is not made with natural rubber latex, DEHP, or PVC.
Storage
Store at 20°C to 25°C (68°F to 77°F). [See USP Controlled Room Temperature.] Protect from freezing.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Magnesium Sulfate in 5% Dextrose Injection is typically administered to pregnant women in emergent situations. When feasible, advise the patient and family of the following:
Fetal-Neonatal Toxicity Reported With Prolonged Use
Continuous administration of Magnesium Sulfate in 5% Dextrose Injection in
pregnant women beyond 5 to 7 days can lead to hypocalcemia and bone
abnormalities in the developing fetus, including skeletal demineralization and
osteopenia. In addition, cases of neonatal fracture have been reported [see Warnings and Precautions (5.1)].
Risk of Magnesium Toxicity
Pregnant women receiving Magnesium Sulfate in 5% Dextrose Injection are at
risk for magnesium toxicity, including facial edema, diminished strength of
deep tendon reflexes, and respiratory depression [see Warnings and Precautions (5.2)].
Baxter Logo
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in Spain
Baxter and Viaflo are trademarks of Baxter International Inc. or its subsidiaries.
SA-30-02-892
