DG Health Cough DM
Dolgencorp, LLC Cough DM Drug Facts
2f22f1bd-3f8e-8a8f-e063-6294a90a8a24
HUMAN OTC DRUG LABEL
Sep 15, 2025
Sixarp, LLC
DUNS: 016329513
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
dextromethorphan polistirex
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (19)
Drug Labeling Information
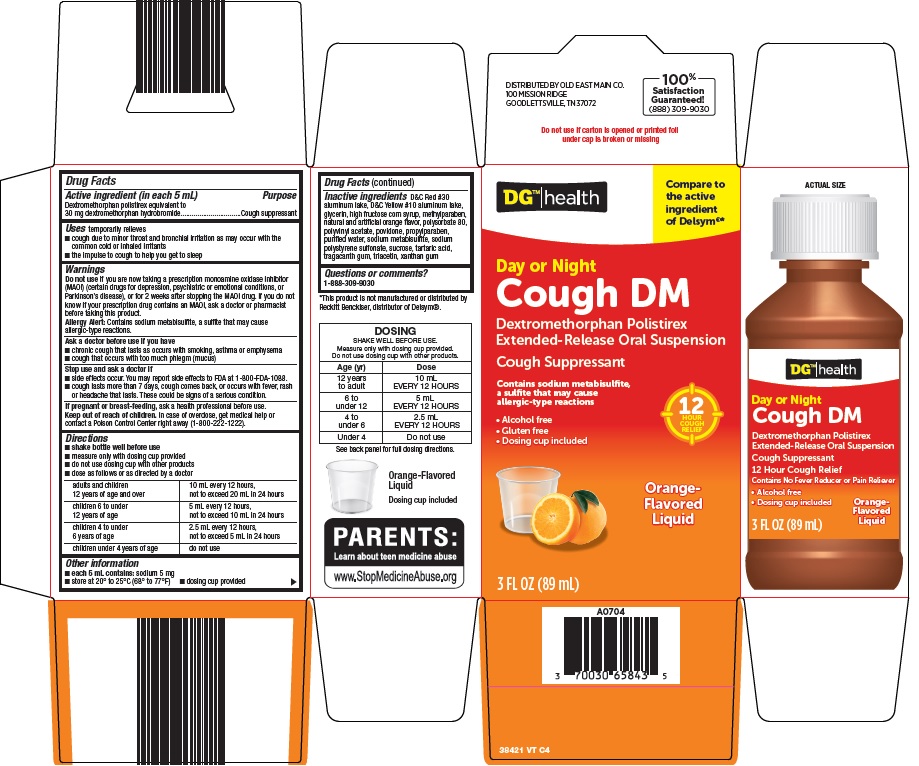
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Principal Display Panel
Compare to the active ingredient of Delsym ®
Day or Night
Cough DM
Dextromethorphan Polistirex Extended-Release Oral Suspension
Cough Suppressant
Contains sodium metabisulfite, a sulfite that may cause allergic-type reactions
12 HOUR COUGH RELIEF
Alcohol free
Gluten free
Dosing cup included
Orange-Flavored Liquid
3 FL OZ (89 mL)
INDICATIONS & USAGE SECTION
Uses
temporarily relieves
- cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants
- the impulse to cough to help you get to sleep
OTC - ACTIVE INGREDIENT SECTION
Active ingredient (in each 5 mL)
Dextromethorphan polistirex equivalent to 30 mg dextromethorphan hydrobromide
OTC - PURPOSE SECTION
Purpose
Cough suppressant
WARNINGS SECTION
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
**Allergy Alert:**Contains sodium metabisulfite, a sulfite that may cause allergic-type reactions.
Ask a doctor before use if you have
- chronic cough that lasts as occurs with smoking, asthma or emphysema
- cough that occurs with too much phlegm (mucus)
Stop use and ask a doctor if
- side effects occur. You may report side effects to FDA at 1-800-FDA-1088.
- cough lasts more than 7 days, cough comes back, or occurs with fever, rash or headache that lasts. These could be signs of a serious condition.
If pregnant or breast-feeding,
ask a health professional before use.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222).
DOSAGE & ADMINISTRATION SECTION
Directions
*shake bottle well before use
- measure only with dosing cup provided
- do not use dosing cup with other products
- dose as follows or as directed by doctor
|
adults and children 12 years of age and over |
10 mL every 12 hours, not to exceed 20 mL in 24 hours |
|
children 6 to under 12 years of age |
5 mL every 12 hours, not to exceed 10 mL in 24 hours |
|
children 4 to under 6 years of age |
2.5 mL every 12 hours, not to exceed 5 mL in 24 hours |
|
children under 4 years of age |
do not use |
SPL UNCLASSIFIED SECTION
Other information
***each 5 mL contains:**sodium 5 mg
- store at 20° to 25°C (68° to 77°F)
- dosing cup provided
INACTIVE INGREDIENT SECTION
Inactive ingredients
D&C Red #30 aluminum lake, D&C Yellow #10 aluminum lake, glycerin, high fructose corn syrup, methylparaben, natural and artificial orange flavor, polysorbate 80, polyvinyl acetate, povidone, propylparaben, purified water, sodium metabisulfite, sodium polystyrene sulfonate, sucrose, tartaric acid, tragacanth gum, triacetin, xanthan gum
OTC - QUESTIONS SECTION
Questions or comments?
1-888-309-9030
