Manufacturing Establishments (1)
Advanced Rx Pharmacy of Tennessee, LLC
117023142
Products (1)
Doxepin Hydrochloride
80425-0316
ANDA213063
ANDA (C73584)
ORAL
April 10, 2023
Drug Labeling Information
DESCRIPTION SECTION
Doxepin hydrochloride is one of a class of psychotherapeutic agents known as dibenzoxepin tricyclic compounds. The molecular formula of the compound is C19H21NO ∙ HCl having a molecular weight of 315.84. It is a white crystalline powder freely soluble in water, in ethanol (96%), and methylene chloride. It may be represented by the following structural formula:
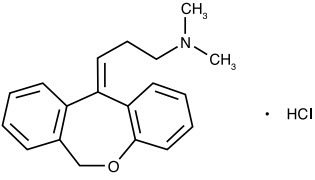
Chemically, doxepin hydrochloride is a dibenzoxepin derivative and is the first of a family of tricyclic psychotherapeutic agents. Specifically, it is an isomeric mixture of 1-Propanamine, 3-dibenz[b,e] oxepin-11 (6H)ylidene-N,N-dimethyl-hydrochloride.
Each 10 mg, 25 mg, 50 mg, 75 mg and 100 mg doxepin capsule for oral administration contains doxepin hydrochloride, USP equivalent to 10 mg, 25 mg, 50 mg, 75 mg and 100 mg of doxepin, respectively and the following inactive ingredients: colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, pregelatinized starch and sodium lauryl sulfate. The empty gelatin capsule shells contain gelatin, sodium lauryl sulfate and titanium dioxide. In addition, the 10 mg empty gelatin capsule shells contain iron oxide red and iron oxide yellow, the 25 mg and 50 mg empty gelatin capsule shells contain D&C Yellow 10 and FD&C Yellow 6, and the 75 mg and 100 mg empty gelatin capsule shells contain D&C Yellow 10 and FD&C Blue 1.
The imprinting ink may contain ferrosoferric oxide, potassium hydroxide, propylene glycol and shellac glaze.
CLINICAL PHARMACOLOGY SECTION
Chemically, doxepin hydrochloride is a dibenzoxepin derivative and is the first of a family of tricyclic psychotherapeutic agents. Specifically, it is an isomeric mixture of 1-Propanamine, 3-dibenz[b,e] oxepin-11 (6H)ylidene-N,N-dimethyl-hydrochloride.
Each 10 mg, 25 mg, 50 mg, 75 mg and 100 mg doxepin capsule for oral administration contains doxepin hydrochloride, USP equivalent to 10 mg, 25 mg, 50 mg, 75 mg and 100 mg of doxepin, respectively and the following inactive ingredients: colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, pregelatinized starch and sodium lauryl sulfate. The empty gelatin capsule shells contain gelatin, sodium lauryl sulfate and titanium dioxide. In addition, the 10 mg empty gelatin capsule shells contain iron oxide red and iron oxide yellow, the 25 mg and 50 mg empty gelatin capsule shells contain D&C Yellow 10 and FD&C Yellow 6, and the 75 mg and 100 mg empty gelatin capsule shells contain D&C Yellow 10 and FD&C Blue 1.
The imprinting ink may contain ferrosoferric oxide, potassium hydroxide, propylene glycol and shellac glaze.
BOXED WARNING SECTION
ADVERSE REACTIONS SECTION
NOTE: Some of the adverse reactions noted below have not been specifically reported with doxepin use. However, due to the close pharmacological similarities among the tricyclics, the reactions should be considered when prescribing doxepin.
Anticholinergic Effects
Dry mouth, blurred vision, constipation and urinary retention have been reported. If they do not subside with continued therapy or become severe, it may be necessary to reduce the dosage.
Central Nervous System Effects
Drowsiness is the most commonly noticed side effect. This tends to disappear as therapy is continued. Other infrequently reported CNS side effects are confusion, disorientation, hallucinations, numbness, paresthesias, ataxia, extrapyramidal symptoms, seizures, tardive dyskinesia and tremor.
Cardiovascular
Cardiovascular effects including hypotension, hypertension and tachycardia have been reported occasionally.
Allergic
Skin rash, edema, photosensitization and pruritus have occasionally occurred.
Hematologic
Eosinophilia has been reported in a few patients. There have been occasional reports of bone marrow depression manifesting as agranulocytosis, leukopenia, thrombocytopenia and purpura.
Gastrointestinal
Nausea, vomiting, indigestion, taste disturbances, diarrhea, anorexia and aphthous stomatitis have been reported. (See ANTICHOLINERGIC EFFECTS.)
Endocrine
Raised or lowered libido, testicular swelling, gynecomastia in males, enlargement of breasts and galactorrhea in the female, raising or lowering of blood sugar levels and syndrome of inappropriate antidiuretic hormone secretion have been reported with tricyclic administration.
Other
Dizziness, tinnitus, weight gain, sweating, chills, fatigue, weakness, flushing, jaundice, alopecia, headache, exacerbation of asthma, angle closure glaucoma, mydriasis and hyperpyrexia (in association with chlorpromazine) have been occasionally observed as adverse effects.
Withdrawal Symptoms
The possibility of development of withdrawal symptoms upon abrupt cessation of treatment after prolonged doxepin administration should be borne in mind. These are not indicative of addiction and gradual withdrawal of medication should not cause these symptoms.
OVERDOSAGE SECTION
Deaths may occur from overdosage with this class of drugs. Multiple drug ingestion (including alcohol) is common in deliberate tricyclic antidepressant overdose. As the management is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment. Signs and symptoms of toxicity develop rapidly after tricyclic antidepressant overdose; therefore, hospital monitoring is required as soon as possible.
Manifestations
Critical manifestations of overdose include: cardiac dysrhythmias, severe hypotension, convulsions and CNS depression, including coma. Changes in the electrocardiogram, particularly in QRS axis or width, are clinically significant indicators of tricyclic antidepressant toxicity.
Other signs of overdose may include: confusion, disturbed concentration, transient visual hallucinations, dilated pupils, agitation, hyperactive reflexes, stupor, drowsiness, muscle rigidity, vomiting, hypothermia, hyperpyrexia, or any of the symptoms listed under ADVERSE REACTIONS.
Deaths have been reported involving overdoses of doxepin.
General Recommendations
General
Obtain an ECG and immediately initiate cardiac monitoring. Protect the patient's airway, establish an intravenous line and initiate gastric decontamination. A minimum of 6 hours of observation with cardiac monitoring and observation for signs of CNS or respiratory depression, hypotension, cardiac dysrhythmias and/or conduction blocks, and seizures is strongly advised. If signs of toxicity occur at any time during this period, extended monitoring is recommended. There are case reports of patients succumbing to fatal dysrhythmias late after overdose; these patients had clinical evidence of significant poisoning prior to death and most received inadequate gastrointestinal decontamination. Monitoring of plasma drug levels should not guide management of the patient.
Gastrointestinal Decontamination
All patients suspected of tricyclic antidepressant overdose should receive gastrointestinal decontamination. This should include large volume gastric lavage followed by activated charcoal. If consciousness is impaired, the airway should be secured prior to lavage. Emesis is contraindicated.
Cardiovascular
A maximal limb lead QRS duration of ≥ 0.10 seconds may be the best indication of the severity of the overdose. Intravenous sodium bicarbonate should be used to maintain the serum pH in the range of 7.45 to 7.55. If the pH response is inadequate, hyperventilation may also be used. Concomitant use of hyperventilation and sodium bicarbonate should be done with extreme caution, with frequent pH monitoring. A pH > 7.60 or a pCO2 < 20 mm Hg is undesirable. Dysrhythmias unresponsive to sodium bicarbonate therapy/hyperventilation may respond to lidocaine, bretylium or phenytoin. Type 1A and 1C antiarrhythmics are generally contraindicated (e.g., quinidine, disopyramide and procainamide).
In rare instances, hemoperfusion may be beneficial in acute refractory cardiovascular instability in patients with acute toxicity. However, hemodialysis, peritoneal dialysis, exchange transfusions and forced diuresis generally have been reported as ineffective in tricyclic antidepressant poisoning.
CNS
In patients with CNS depression, early intubation is advised because of the potential for abrupt deterioration. Seizures should be controlled with benzodiazepines, or if these are ineffective, other anticonvulsants (e.g., phenobarbital, phenytoin). Physostigmine is not recommended except to treat life threatening symptoms that have been unresponsive to other therapies, and then only in consultation with a poison control center.
Psychiatric Follow-up
Since overdosage is often deliberate, patients may attempt suicide by other means during the recovery phase. Psychiatric referral may be appropriate.
Pediatric Management
The principles of management of child and adult overdosages are similar. It is strongly recommended that the physician contact the local poison control center for specific pediatric treatment.
