Numbing Cream
Initial Drug Listing - 4% lidocaine numbing cream
359884d6-188a-e503-e063-6294a90acab4
HUMAN OTC DRUG LABEL
May 20, 2025
100 KARMA INC
DUNS: 120186232
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
LIDOCAINE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
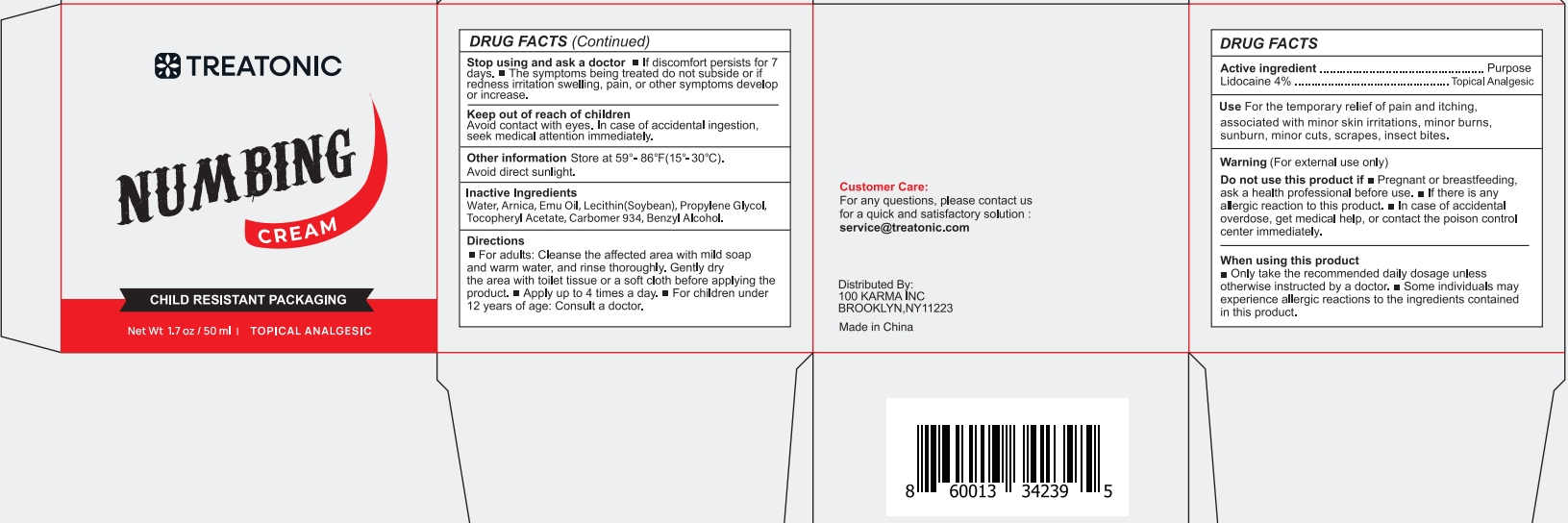
INDICATIONS & USAGE SECTION
For the temporary relief of pain and itching!associated with minor skin irritations, minor burnssunburn,minor cuts,scrapes, insect bites.
OTC - ACTIVE INGREDIENT SECTION
4% lidocaine
OTC - PURPOSE SECTION
topical analgesic
WARNINGS SECTION
For external use only
Do not use this product if
-
Pregnant or breastfeeding, ask a health professional before use.
-
If there is any allergic reaction to this product.
-
In case of accidental overdose, get medical help, or contact the poison control center immediately.
-
Only take the recommended daily dosage unless otherwise instructed by a doctor.
-
Some individuals may experience allergic reactions to the ingredients contained in this product.
Stop using and ask a doctor
- If discomfort persists for 7 days.
- The symptoms being treated do not subside or if redness irritation swelling, pain, or other symptoms develop or increase.
Keep out of reach of children Avoid contact with eyes.
In case of accidental ingestion, seek medical attention immediately.
DOSAGE & ADMINISTRATION SECTION
- For adults: Cleanse the affected area with mild soap and warm water, and rinse thoroughly. Gently dry the area with toilet tissue or a soft cloth before applying the product.
- Apply up to 4 times a day.
- For children under 12 years of age: Consult a doctor.
OTHER SAFETY INFORMATION
Store at 59°-86°F (15°-30°C). Avoid direct sunlight.
INACTIVE INGREDIENT SECTION
Water, Arnica, Emu Oil, Lecithin(Soybean), Propylene Glycol,Tocopheryl Acetate, Carbomer 934, Benzyl Alcohol.
