OTOVEL
These highlights do not include all the information needed to use OTOVEL safely and effectively. See full prescribing information for OTOVEL. OTOVEL (ciprofloxacin and fluocinolone acetonide) otic solutionInitial U.S. Approval: 2016
1a7e4806-3138-4b7a-b798-a99e163ee846
HUMAN PRESCRIPTION DRUG LABEL
Sep 12, 2019
Arbor Pharmaceuticals
DUNS: 781796417
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
(ciprofloxacin and fluocinolone acetonide)
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
SPL PATIENT PACKAGE INSERT SECTION
PATIENT INFORMATION
OTOVEL (OH-toe-vel)
(ciprofloxacin and fluocinolone acetonide)
otic solution
What is OTOVEL?
OTOVEL is a prescription medicine used in the ear only (otic use) that contains 2 medicines, a quinolone antibiotic medicine called ciprofloxacin and a corticosteroid medicine called fluocinolone acetonide. OTOVEL is used in children 6 months of age and older to treat a type of middle ear infection called acute otitis media with tympanostomy tubes (AOMT) in children who have a tube in their eardrum known as a tympanostomy tube, to prevent having too much fluid in the middle ear.
It is not known if OTOVEL is safe and effective in children under 6 months of age.
Who should not use** OTOVEL?**
Do not use OTOVEL if you:
- Are allergic to ciprofloxacin, quinolones, fluocinolone acetonide, corticosteroids or any of the ingredients in OTOVEL. See the end of this Patient Information leaflet for a complete list of ingredients in OTOVEL.
- Have an outer ear canal infection caused by certain viruses including chicken pox (varicella) and the herpes simplex virus,
- Have an ear infection caused by a fungus.
What should I tell my healthcare provider before using OTOVEL?
Before using OTOVEL, tell your healthcare provider about all of your medical conditions, including if you:
- Are pregnant or plan to become pregnant, although OTOVEL is not expected to harm your baby.
- Are breastfeeding or plan to breastfeed, although OTOVEL is not expected to pass into your breast milk and to harm your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use OTOVEL?
- Read the detailed Instructions for Use that come with OTOVEL.
- Use OTOVEL exactly as your healthcare provider tells you to use it. *OTOVEL is for use in the ear only (otic use). Do not inject OTOVEL or use OTOVEL in the eye.
- OTOVEL comes as a liquid in single-dose vials.
- Apply the entire dose of OTOVEL from 1 of the single-dose vials, into the affected ear 2 times a day (for a total of 2 single-dose vials a day) for 7 days. Each dose should be about 12 hours apart.
If your symptoms do not improve after 7 days of treatment with OTOVEL, call your healthcare provider.
- Call your healthcare provider right away if:
- you have fluid that continues to drain from your ear (otorrhea) after you have finished your treatment with OTOVEL,
- you have fluid that drains from your ear 2 or more times within 6 months after you stop treatment with OTOVEL.
What are the possible side effects of****OTOVEL?
OTOVEL may cause serious side effects, including:
*Allergic reactions. Stop using OTOVEL and call your healthcare provider if you have any of the following signs or symptoms of an allergic reaction:
- hives (urticaria)
- swelling of your face, lips, mouth, or tongue
- rash
- itching
- trouble breathing
- dizziness, fast heartbeat, or pounding in your chest
The most common side effects that occurred during the testing of OTOVEL include:
- fluid that continues to drain from your ear (otorrhea)
- extra tissue that grows on a part of the ear that has been injured (excessive granulation tissue)
- ear pain
- ear infection
- ear itching (pruritus)
- swelling of the outer or inside part of the ear
- balance problems
If an allergic reaction to OTOVEL occurs, stop using the product and contact your doctor.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of OTOVEL. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store OTOVEL?
- Store unopened OTOVEL vials in the protective foil pouch they come in.
- Store OTOVEL at 20°-25°C (68°-77°F).
- Keep OTOVEL out of light.
- Do not open the OTOVEL foil pouch until ready to use.
- When the OTOVEL foil pouch is opened, use the vials within 7 days.
- When an OTOVEL vial is opened, use it right away.
Keep OTOVEL and all medicines out of the reach of children.
General information about the safe and effective use of OTOVEL.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use OTOVEL for a condition for which it was not prescribed. Do not give OTOVEL to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about OTOVEL. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about OTOVEL that is written for healthcare professionals.
What are the ingredients in OTOVEL?
Active ingredients: ciprofloxacin and fluocinolone acetonide.
Inactive ingredients: polysorbate, povidone, glycerin, and water.
Distributed by: Arbor Pharmaceuticals, LLC, Atlanta, GA 30328.
Under license of Laboratorios SALVAT, S.A. OTOVEL is a registered trademark of Laboratorios SALVAT, S.A.
For more information, go to www.arborpharma.com or call 1-866-516-4950.
This Patient Information has been approved by the U.S. Food and Drug Administration Issued: 4/2016
INSTRUCTIONS FOR USE SECTION
INSTRUCTIONS FOR USE
OTOVEL (OH-toe-vel)
(ciprofloxacin and fluocinolone acetonide)
otic solution
Read this Instructions for Use that comes with OTOVEL before you start using it and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment.
Important information about OTOVEL:
*OTOVEL is for use in the ear only (otic use). Do not inject OTOVEL or use OTOVEL in the eye.
- Use OTOVEL exactly as your healthcare provider tells you to use it.
How should I use OTOVEL?
|
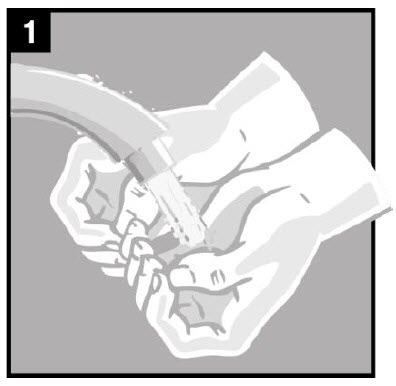
Step 1. You or your caregiver should wash their hands |
|
|
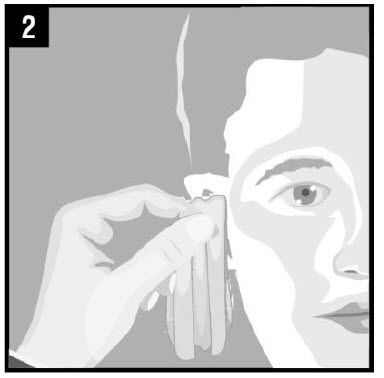
Step 2. Gently clean any fluid (discharge) from the |
|
|
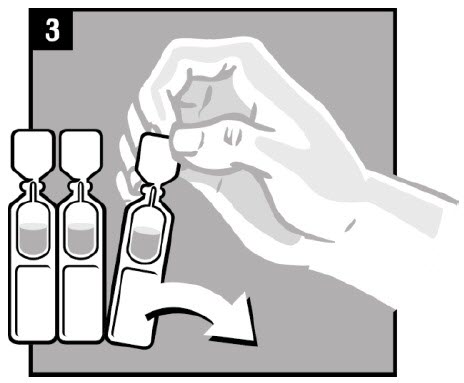
Step 3. Remove OTOVEL from the protective foil |
|
|
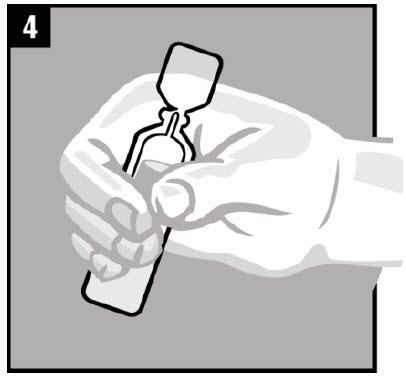
Step 4. Warm the dose of OTOVEL by holding the vial |
|
|
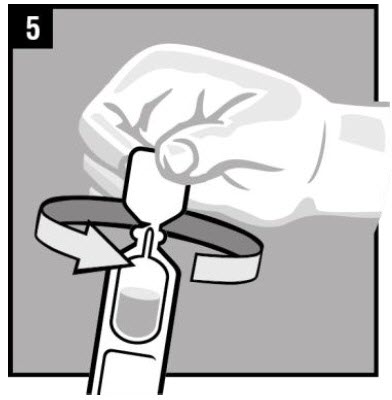
Step 5. Twist off the vial cap in the direction of the |
|
|
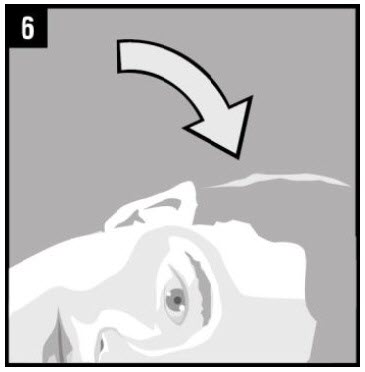
Step 6. The person receiving OTOVEL should be on |
|
|
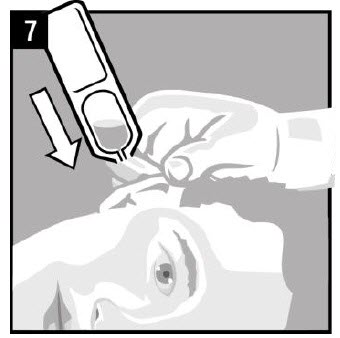
Step 7. Hold the vial of OTOVEL in your hand and |
|
|
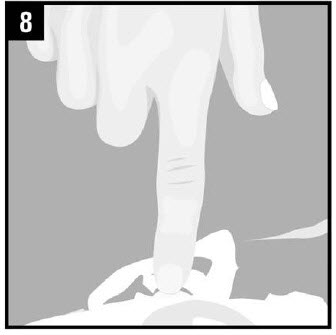
Step 8. Gentlypress the part of the ear known |
|
|
Step 9. Remain on your side with the affected |
|
|
Step 10. If your healthcare provider has told you | |
|
Step 11. Safely throw away OTOVEL vials after |
This Instructions for Use has been approved by the Food and Drug Administration.