Emetrol Cherry
Emetrol
a8469128-592a-4682-82c7-4d2a6f97a998
HUMAN OTC DRUG LABEL
Jun 10, 2025
WellSpring Pharmaceutical Corporation
DUNS: 110999054
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
phosphorated carbohydrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
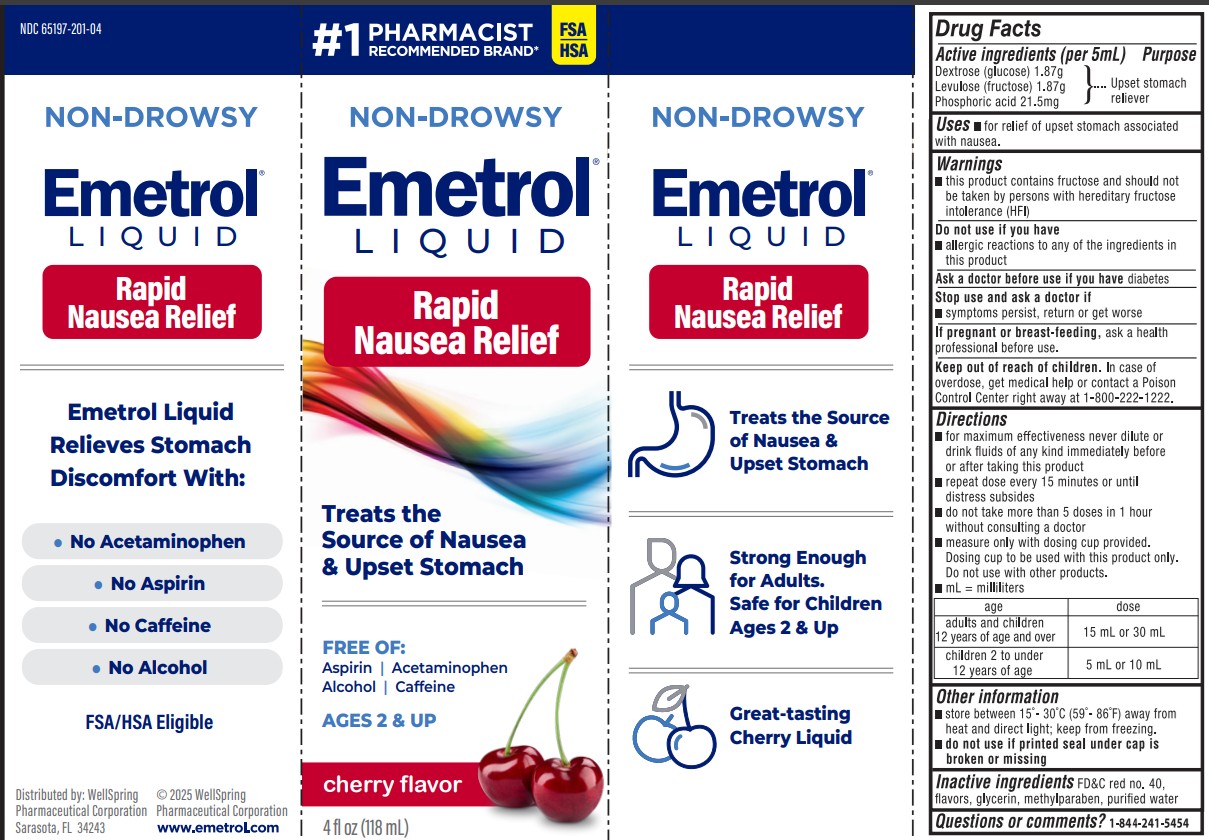
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL
Emetrol Loquid Rapid Nausea Relief
Treats the source of Nausea & upset stomach
CT201048H

Emetrol 12 oz
INDICATIONS & USAGE SECTION
Uses
For relief of upset stomach associated with nausea due to overindulgence in food and drink.
OTC - ACTIVE INGREDIENT SECTION
Active ingredients
Phosphorated carbohydrate solution*
*each 5 mL contains:
- 1.87 g Dextrose (glucose)
- 1.87 g Levulose (fructose)
- 21.5 mg Phosphoric acid
OTC - PURPOSE SECTION
Purpose
Upset Stomach Reliever
WARNINGS SECTION
Warnings
- This product contains fructose and should not be taken by persons with hereditary fructose intolerance (HFI).
Do not use if you have
- allergic reactions to any of the ingredients in this product
Ask a doctor before use if you have
- diabetes
Stop use and ask a doctor if
- symptoms persist, return or get worse
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
DOSAGE & ADMINISTRATION SECTION
Directions
- for maximum effectiveness never dilute or drink fluids of any kind immediately before or after taking this product
- adults and children 12 years of age and over: one to two tablespoons
- children 2 to under 12: one or two teaspoons
- repeat dose every 15 minutes or until distress subsides
- do not take more than 5 doses in 1 hour without consulting a doctor
- measure only with dosing cup provided. Dosing cup to be used with Emetrol only. Do not use with other products.
SPL UNCLASSIFIED SECTION
Distributed By
WellSpring Pharmaceutical Corporation
Sarasota, FL 34243 USA
(c) WellSpring 2019
INACTIVE INGREDIENT SECTION
Inactive ingredients
FD&C red no. 40, flavors, glycerin, methylparaben, and purified water.
