Ethambutol Hydrochloride
Ethambutol Hydrochloride Tablets USP Rx Only 8228001/0825(F)
d32e9865-8edd-4644-9939-62ed119db3ef
HUMAN PRESCRIPTION DRUG LABEL
Sep 18, 2025
American Health Packaging
DUNS: 929561009
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Ethambutol Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Display Panel — Blister — 400 mg
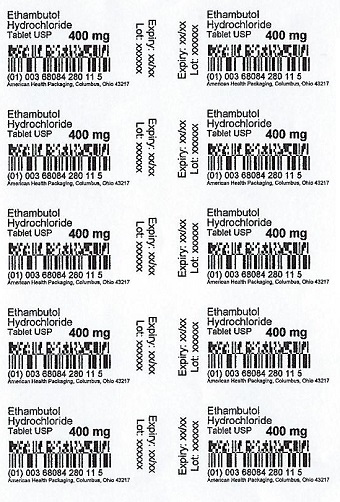
Ethambutol
Hydrochloride
Tablets USP
400 mg
SPL UNCLASSIFIED SECTION
PACKAGING INFORMATION
American Health Packaging unit dose blisters (see How Supplied section)
contain drug product from Lupin Pharmaceuticals, Inc. as follows:
(400 mg / 100 UD) NDC 68084-280-01 packaged from NDC 68180-281
Distributed by:
American Health Packaging
Columbus, OH 43217
8228001/0825(F)
DESCRIPTION SECTION
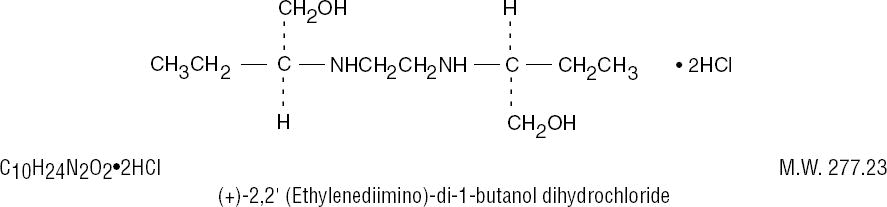
DESCRIPTION
Ethambutol hydrochloride is an oral chemotherapeutic agent which is specifically effective against actively growing microorganisms of the genus Mycobacterium, including M. tuberculosis. Ethambutol hydrochloride is a white, crystalline powder. It is freely soluble in water; soluble in alcohol and in methanol. The structural formula is:
Each tablet, for oral administration, contains 100 mg or 400 mg Ethambutol Hydrochloride. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, corn starch, magnesium stearate, povidone and talc. Film coating contains: ethyl cellulose, hypromellose, macrogol, propylene glycol, talc and titanium dioxide.
HOW SUPPLIED SECTION
HOW SUPPLIED
Ethambutol Hydrochloride Tablets USP, 400 mg are available as white to off- white, round, biconvex, film-coated tablets debossed with 'L' and 'U' on either side of the breakline on one side and 'C32' on other side.
They are supplied as follows:
Unit dose packages of 100 (10 x 10) NDC 68084-280-01
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Protect from light and moisture.
FOR YOUR PROTECTION: Do not use if blister is torn or broken.
LUPIN and the  are registered trademarks of Lupin Pharmaceuticals, Inc.
are registered trademarks of Lupin Pharmaceuticals, Inc.
