Exchange Select Antacid
Xiamen-AAFES Soft Chews
3de81025-b81c-374a-e063-6394a90a00cf
HUMAN OTC DRUG LABEL
Sep 3, 2025
Xiamen Kang Zhongyuan Biotechnology Co., Ltd.
DUNS: 411759931
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Calcium Carbonate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
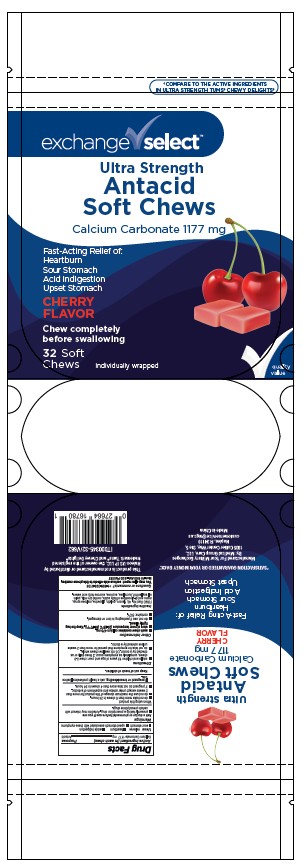
INDICATIONS & USAGE SECTION
Uses
Relieves
- heartburn
- acid indigestion
- sour stomach
- upset stomach associated with these symptoms
OTC - ACTIVE INGREDIENT SECTION
Active ingredient (in each chew)
Calcium carbonate 1,177 mg
OTC - PURPOSE SECTION
Purpose
Antacid
WARNINGS SECTION
Warnings
Ask a doctor or pharmacist before use if you are
- presently taking a prescription drug. Antacids may interact with certain perscription drugs.
OTC - WHEN USING SECTION
When using this product
- do not take more than 6 chews in 24 hours
- do not use the maximum dosage of this product for more than 2 weeks except under the advice and supervision of a doctor
- if pregnant do not take more than 4 chews in 24 hours
OTC - PREGNANCY OR BREAST FEEDING SECTION
If pregnant or breast-feeding, ask a health professional before use
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children.
DOSAGE & ADMINISTRATION SECTION
Directions
- Adults and children 12 years of age and over: chew 2-3 chews, completely as symptoms occur, 2 times daily or as directed by a doctor. Do not swallow chews whole.
- Do not take for symptoms that persist for mare than 2 weekis unless advised by a doctor
OTHER SAFETY INFORMATION
Other information
***Each chew contains:**calcium 470 mg
- Store at room temperatrue 20-25ºC (68-77ºF). Keep bag tightly closed.
- Do not use if packaging is torn or damaged.
- Contains: soy
INACTIVE INGREDIENT SECTION
Inactive ingredients:
FD&C Red No. 40, Flaors, Gelatin, Glycerin, Soy Lecithin, Maltose Syrup, Mono and Di-glycerides of Fatty Acids, Nonfat dry milk, Palm Oil, Sucralose, Sucrose Fatty Acid Esters.
OTC - QUESTIONS SECTION
Questions or comments? (866) 306-9136
You may also report serious side effects to this phone number.
Mon-Fri 9:00 a.m - 5:00 p.m ET
