Systane Ultra
0537c23a-15f7-be64-1231-a6c93468e9a8
HUMAN OTC DRUG LABEL
Jul 30, 2025
Alcon Laboratories, Inc.
DUNS: 008018525
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Polyethylene Glycol 400 and Propylene Glycol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
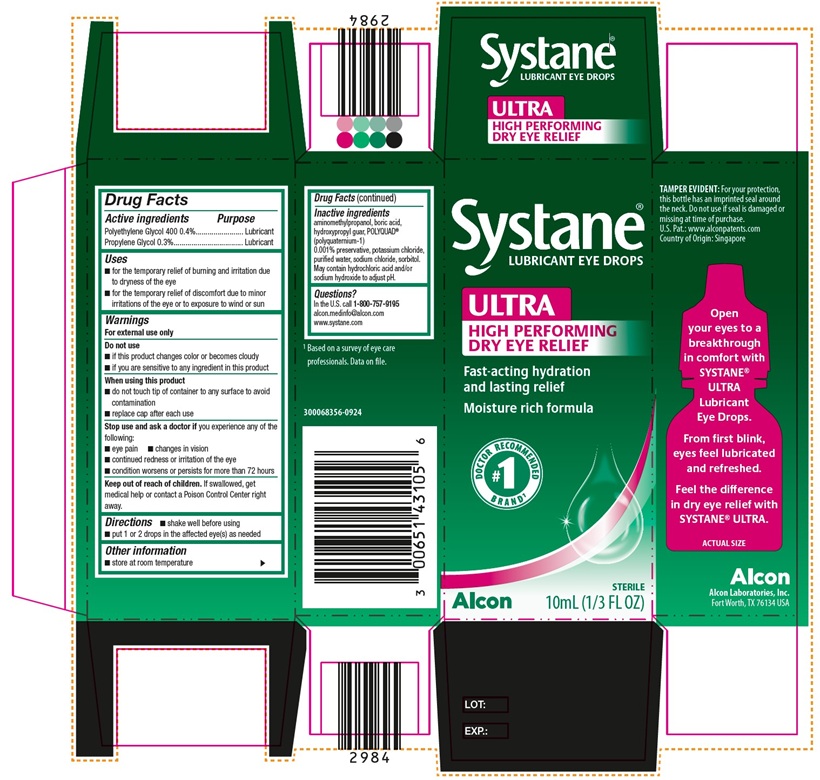
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
Systane**®******
****LUBRICANT EYE DROPS
ULTRA
HIGH PERFORMING
DRY EYE RELIEF
Fast-acting hydration
and lasting relief
Moisture rich formula
** #1 Doctor Recommended Brand**1****
****Alcon STERILE
10mL (1/3 FL OZ)
SIDE PANEL:
TAMPER EVIDENT: For your protection, this bottle has an imprinted seal around the neck. Do not use if seal is damaged or missing at time of purchase.
U.S. Pat.: www.alconpatents.com
Country Of Origin: Singapore
Open your eyes to a breakthrough in comfort with Systane® ULTRA Lubricant Eye Drops.
From first blink, eyes feel lubricated and refreshed. Feel the difference in dry eye relief with SYSTANE® ULTRA.
ACTUAL SIZE
Alcon
Alcon Laboratories, Inc.
Fort Worth, TX 76134 USA
1 Based on a survey of eye care professionals. Data on file.
300068356-0924
Systane**®******
****LUBRICANT EYE DROPS
ULTRA
HIGH PERFORMING
DRY EYE RELIEF
Fast-acting hydration
and lasting relief
Moisture rich formula
#1 Doctor Recommended Brand****1Alcon STERILE
10mL (1/3 FL OZ)
SIDE PANEL:
TAMPER EVIDENT: For your protection, this bottle has an imprinted seal around the neck. Do not use if seal is damaged or missing at time of purchase.
www.alconpatents.com
Made in USA from domestic and imported materials.
Open your eyes to a breakthrough in comfort with Systane® ULTRA Lubricant Eye Drops.
From first blink, eyes feel lubricated and refreshed.
Feel the difference in dry eye relief with SYSTANE® ULTRA.
ACTUAL SIZE
Alcon
Alcon Laboratories, Inc.
Fort Worth, TX 76134 USA
1Based on a survey of eye care professionals. Data on file.
300051246-1221

INDICATIONS & USAGE SECTION
Uses
- for the temporary relief of burning and irritation due to dryness of the eye
- for the temporary relief of discomfort due to minor irritations of the eye or to exposure to wind or sun
INACTIVE INGREDIENT SECTION
Inactive ingredients
aminomethylpropanol, boric acid, hydroxypropyl guar, POLYQUAD® (polyquarternium-1) 0.001% preservative, potassium chloride, purified water, sodium chloride, sorbitol. May contain hydrochloric acid and/or sodium hydroxide to adjust pH.
OTC - ACTIVE INGREDIENT SECTION
OTC - DO NOT USE SECTION
Do not use
- if this product changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
WARNINGS SECTION
Warnings
For external use only
DOSAGE & ADMINISTRATION SECTION
Directions
- shake well before using
- put 1 or 2 drops in the affected eye(s) as needed
OTC - WHEN USING SECTION
When using this product
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
OTC - STOP USE SECTION
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
OTHER SAFETY INFORMATION
Other information
- store at room temperature
OTC - PURPOSE SECTION
OTC - QUESTIONS SECTION
Questions?
In the U.S.
call1-800-757-9195
alcon.medinfo@alcon.com
www.systane.com
