Flecainide Acetate
Flecainide Acetate Tablets USP
5b33e96a-8336-46c4-aac7-4a6c0039cc80
HUMAN PRESCRIPTION DRUG LABEL
Jan 11, 2024
Bayshore Pharmaceuticals LLC
DUNS: 968737416
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
flecainide acetate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
flecainide acetate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
flecainide acetate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
Flecainide acetate tablets, USP are an antiarrhythmic drug available in tablets of 50, 100, or 150 mg for oral administration. Flecainide acetate is benzamide, N-(2-piperidinylmethyl)-2,5-bis(2,2,2-trifluoroethoxy)-monoacetate. The structural formula is given below.
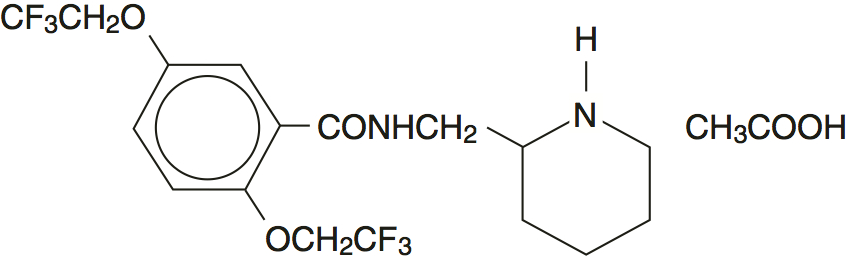
Flecainide acetate is a white crystalline substance with a pKa of 9.3. It has an aqueous solubility of 48.4 mg/mL at 37°C.
Flecainide acetate tablets, USP also contain: croscarmellose sodium, hydrogenated vegetable oil, magnesium stearate, microcrystalline cellulose, and pregelatinized starch.
