Carbamazepine
CARBAMAZEPINE TABLETS USP, 200 mg / CARBAMAZEPINE TABLETS USP (CHEWABLE), 100 mg 0109 0778 Rx only
7f9ed987-5e34-4536-a22f-4898c9797e61
HUMAN PRESCRIPTION DRUG LABEL
Sep 13, 2023
NCS HealthCare of KY, LLC dba Vangard Labs
DUNS: 050052943
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Carbamazepine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL PRINCIPAL DISPLAY PANEL
INDICATIONS & USAGE SECTION
INDICATIONS AND USAGE
Epilepsy
Carbamazepine tablets USP and carbamazepine tablets USP (chewable) are indicated for use as an anticonvulsant drug. Evidence supporting efficacy of carbamazepine tablets USP and carbamazepine tablets USP (chewable) as an anticonvulsant was derived from active drug-controlled studies that enrolled patients with the following seizure types:
1. Partial seizures with complex symptomatology (psychomotor, temporal lobe). Patients with these seizures appear to show greater improvement than those with other types.
2. Generalized tonic-clonic seizures (grand mal).
3. Mixed seizure patterns which include the above, or other partial or generalized seizures. Absence seizures (petit mal) do not appear to be controlled by carbamazepine tablets USP and carbamazepine tablets USP (chewable) (seePRECAUTIONS,General).
Trigeminal Neuralgia
Carbamazepine tablets USP and carbamazepine tablets USP (chewable) are indicated in the treatment of the pain associated with true trigeminal neuralgia.
Beneficial results have also been reported in glossopharyngeal neuralgia.
This drug is not a simple analgesic and should not be used for the relief of trivial aches or pains.
DESCRIPTION SECTION
DESCRIPTION
Carbamazepine, USP is an anticonvulsant and specific analgesic for trigeminal neuralgia, available for oral administration as chewable tablets of 100 mg and tablets of 200 mg. Its chemical name is 5H-dibenz[b,f]azepine-5-carboxamide, and its structural formula is:
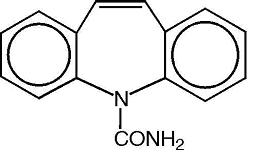
C15H12N2O M.W. 236.27
Carbamazepine, USP is a white to off-white powder, practically insoluble in water and soluble in alcohol and in acetone.
Carbamazepine tablets USP, 200 mg contain the inactive ingredients colloidal silicon dioxide, croscarmellose sodium, ethylcellulose, glycerin, lactose monohydrate, magnesium stearate, and sodium starch glycolate.
Carbamazepine tablets USP (chewable), 100 mg contain the inactive ingredients acacia, colloidal silicon dioxide, croscarmellose sodium, ethylcellulose, FD&C Red #40 aluminum lake, flavoring, glycerin, lactose monohydrate, magnesium stearate, pregelatinized corn starch, and sucrose.
Carbamazepine tablets USP, 200 mg meet USP Dissolution Test 3.
Carbamazepine tablets USP (chewable), 100 mg meet USP Dissolution Test 1.
