Burn Cream
Urgent First Aid Burn Cream
fe439c60-5a02-648f-e053-6294a90a1403
HUMAN OTC DRUG LABEL
Aug 23, 2025
Trifecta Pharmaceuticals USA LLC
DUNS: 079424163
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Benzalkonium Chloride, Lidocaine HCI
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (15)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
LABEL
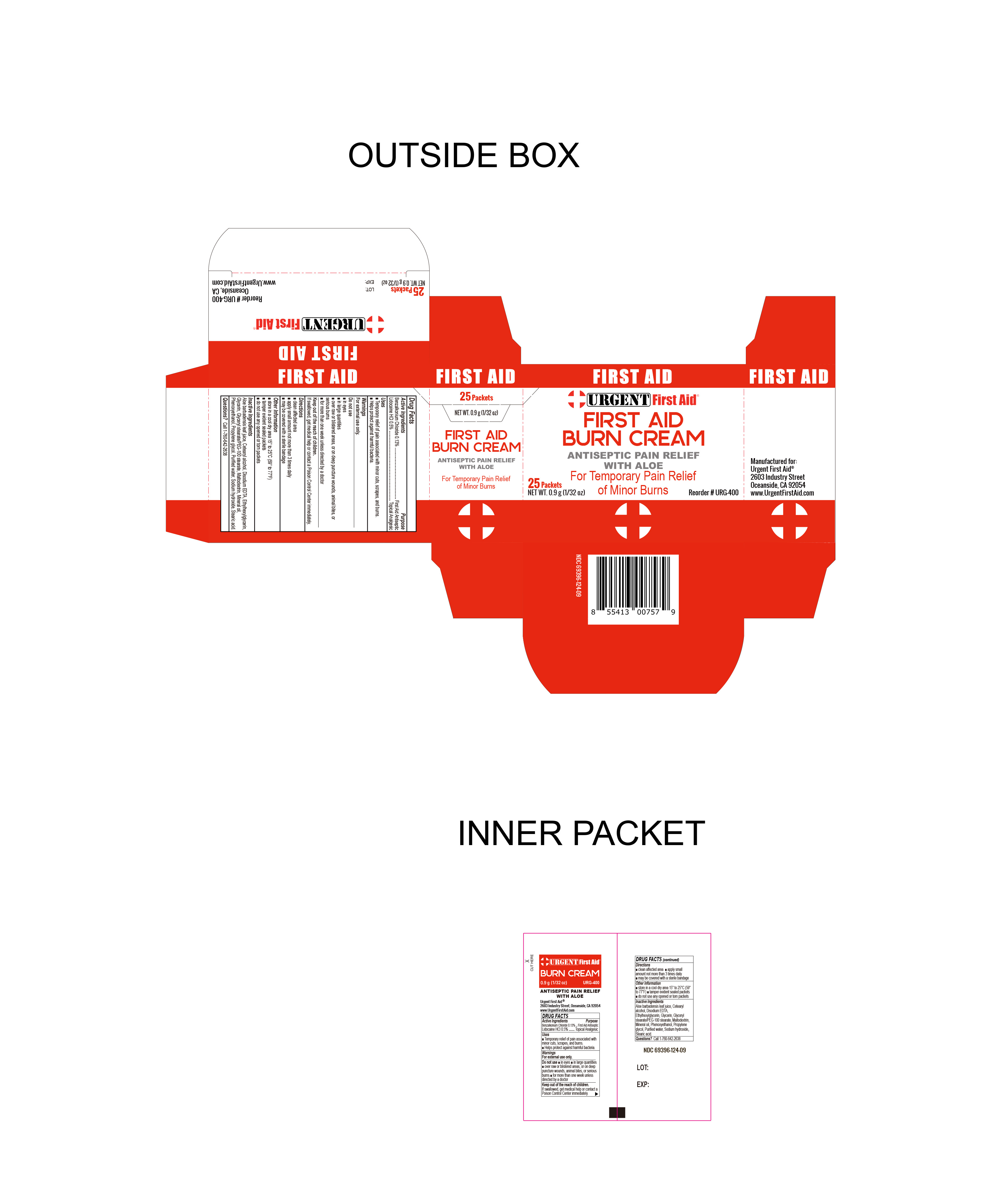
INDICATIONS & USAGE SECTION
Uses
Temporary Relief of pain associated with minor cuts, scrapes and burns.
Helps protect against harmful bacteria.
OTC - ACTIVE INGREDIENT SECTION
Benzalkonium Chloride 0.13%
OTC - PURPOSE SECTION
Purpose
First Aid Antiseptic
WARNINGS SECTION
Warnings
For external Use Only
Do not use
- in eyes
- in large quantities
- over raw or blistered areas, or on deep puncture wounds, animal bites, or serious burns
- for more than one week unless directed by a doctor
DOSAGE & ADMINISTRATION SECTION
Directions
- Clean the affected area
- Apply a small amount not more than 3 times daily
- May be covered with a sterile bandage
INACTIVE INGREDIENT SECTION
Inactive Ingredients
Aloe barbadensis leaf juice, Cetearyl alcohol, Disodium EDTA, Ethylhexylglycerin, Glycerin, Glyceryl stearate/PEG-100 stearate, Maltodextrin, Mineral oil, Phenoxyethanol, Propylene glycol, purified water, stearic acid, Triethanolamine.
OTC - QUESTIONS SECTION
Questions
To reorder:
Call 1-760-642-2638
STORAGE AND HANDLING SECTION
Storage Information
- Store in cool dry area 15° to 25°C (59° to 77°F).
- tamper evident sealed packets
- do not use any opened or torn packets
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center immediately.
SPL UNCLASSIFIED SECTION
Other Information
Manufactured for:
Urgent First Aid®
2603 Industry Street
Oceanside, CA. 92054 USA
www.UrgentFirstAid.com
Reorder # URG-400
