Diaper Rash
HEB Hill Country 020.002/020AC-AD Diaper Rash Ointment 40% Zinc Oxide
f0b31d52-2903-487a-aa70-0dbdf4c4d7e7
HUMAN OTC DRUG LABEL
Sep 3, 2025
H E B
DUNS: 007924756
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Zinc oxide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
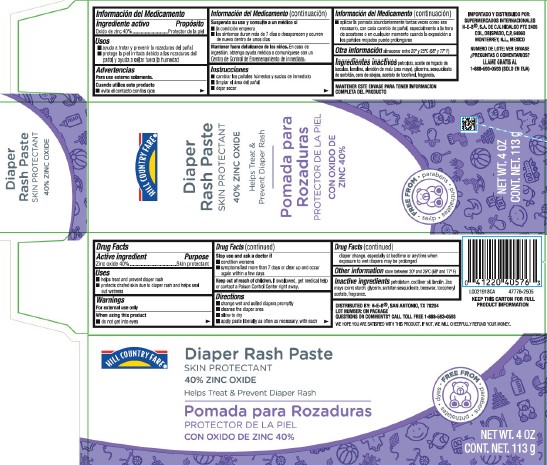
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal display panel
HILL COUNTRY FARE ®
Diaper Rash Paste
SKIN PROTECTANT
40% ZINC OXIDE
Helps Soothe & Prevent Diaper Rash
FREE FROM
- parabens
- phthalates
- dyes
NET WT. 4 OZ
CONT. NET. 113 g
INDICATIONS & USAGE SECTION
Uses
- helps treat and prevent diaper rash
- protects chafed skin due to diaper rash and helps seal out wetness
ADVERSE REACTIONS SECTION
Adverse Reactions
KEEP THIS CARTON FOR FULL PRODUCT INFORMATION
DISTRIBUTED BY: H-E-B ®, SAN ANTONIO, TN 78204
LOT NUMBER: ON PACKAGE
QUESTIONS OR COMMENTS? CALL TOLL FREE 1-888-593-0593
WE HOPE YOU ARE SATISFIED WITH THIS PRODUCT. IF NOT, WE WILL CHEERFULLY REFUND YOUR MONEY.
OTC - STOP USE SECTION
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
- change wet and soilded diapers promptly
- cleanse the diaper area
- allow to dry
- apply paste liberally as often as necessary, with each diaper change, especially at bedtime or anytime when exposure to wet diapers may be prolonged
OTC - ACTIVE INGREDIENT SECTION
Active ingredient
Zinc oxide 40%
OTC - PURPOSE SECTION
Purpose
Skin protectant
WARNINGS SECTION
Warnings
For external use only
OTC - WHEN USING SECTION
When using this product
- do not get into eyes
SPL UNCLASSIFIED SECTION
Other information
store between 20⁰ and 25⁰C (68⁰ and 77⁰F)
INACTIVE INGREDIENT SECTION
Inactive ingredients
petrolatum, cod liver oil, lanolin, Zea mays (corn) starch, glycerin, sorbitan sesquioleate, beeswax, tocopheryl acetate, fragrance
