Calcemin Advance
Calcemin Advance
Approved
Approval ID
3161469e-6402-607a-e054-00144ff88e88
Product Type
HUMAN OTC DRUG LABEL
Effective Date
Sep 16, 2025
Manufacturers
FDA
Bayer HealthCare LLC.
DUNS: 112117283
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Calcium Citrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
NDC Product Code0280-0272
Product Classification
G
Generic Name
Calcium Citrate
Product Specifications
Route of AdministrationORAL
Effective DateSeptember 16, 2025
FDA Product Classification
INGREDIENTS (18)
CALCIUM CARBONATEActive
Quantity: 500 mg in 1 1
Code: H0G9379FGK
Classification: ACTIB
PREVITAMIN D3Active
Quantity: 3 mg in 1 1
Code: HDA46400N5
Classification: ACTIB
MAGNESIUM OXIDEActive
Quantity: 40 mg in 1 1
Code: 3A3U0GI71G
Classification: ACTIB
ZINC OXIDEActive
Quantity: 7.5 mg in 1 1
Code: SOI2LOH54Z
Classification: ACTIB
SODIUM BORATEActive
Quantity: 2.45 mg in 1 1
Code: 91MBZ8H3QO
Classification: ACTIB
CROSCARMELLOSE SODIUMInactive
Code: M28OL1HH48
Classification: IACT
STEARIC ACIDInactive
Code: 4ELV7Z65AP
Classification: IACT
SODIUM LAURYL SULFATEInactive
Code: 368GB5141J
Classification: IACT
HYPROMELLOSESInactive
Code: 3NXW29V3WO
Classification: IACT
TITANIUM DIOXIDEInactive
Code: 15FIX9V2JP
Classification: IACT
TRIACETINInactive
Code: XHX3C3X673
Classification: IACT
MAGNESIUM SILICATEInactive
Code: 9B9691B2N9
Classification: IACT
MINERAL OILInactive
Code: T5L8T28FGP
Classification: IACT
FD&C RED NO. 40Inactive
Code: WZB9127XOA
Classification: IACT
FD&C YELLOW NO. 6Inactive
Code: H77VEI93A8
Classification: IACT
FD&C BLUE NO. 1Inactive
Code: H3R47K3TBD
Classification: IACT
WATERInactive
Code: 059QF0KO0R
Classification: IACT
MALTODEXTRINInactive
Code: 7CVR7L4A2D
Classification: IACT
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
LOINC: 51945-4Updated: 4/1/2016
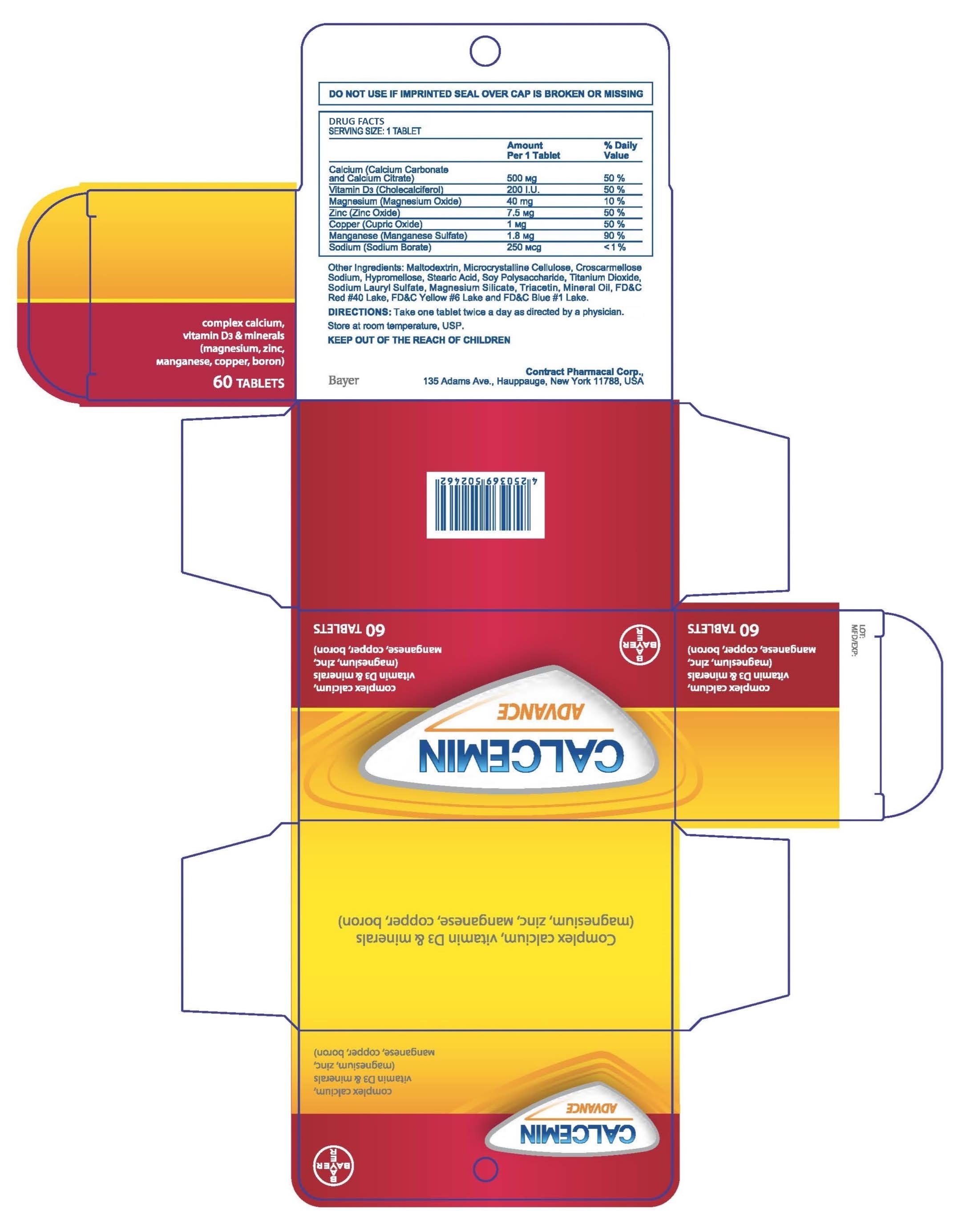
SPL UNCLASSIFIED SECTION
LOINC: 42229-5Updated: 4/1/2016
Drug Facts
Drug Facts
