Nymalize
These highlights do not include all the information needed to use NYMALIZE safely and effectively. See full prescribing information for NYMALIZE. NYMALIZE (nimodipine) oral solution Initial U.S. Approval: 1988
e660fa26-b5c4-4d6d-8887-9e22b6666a10
HUMAN PRESCRIPTION DRUG LABEL
Mar 31, 2023
Azurity Pharmaceuticals, Inc.
DUNS: 117505635
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
nimodipine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
nimodipine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
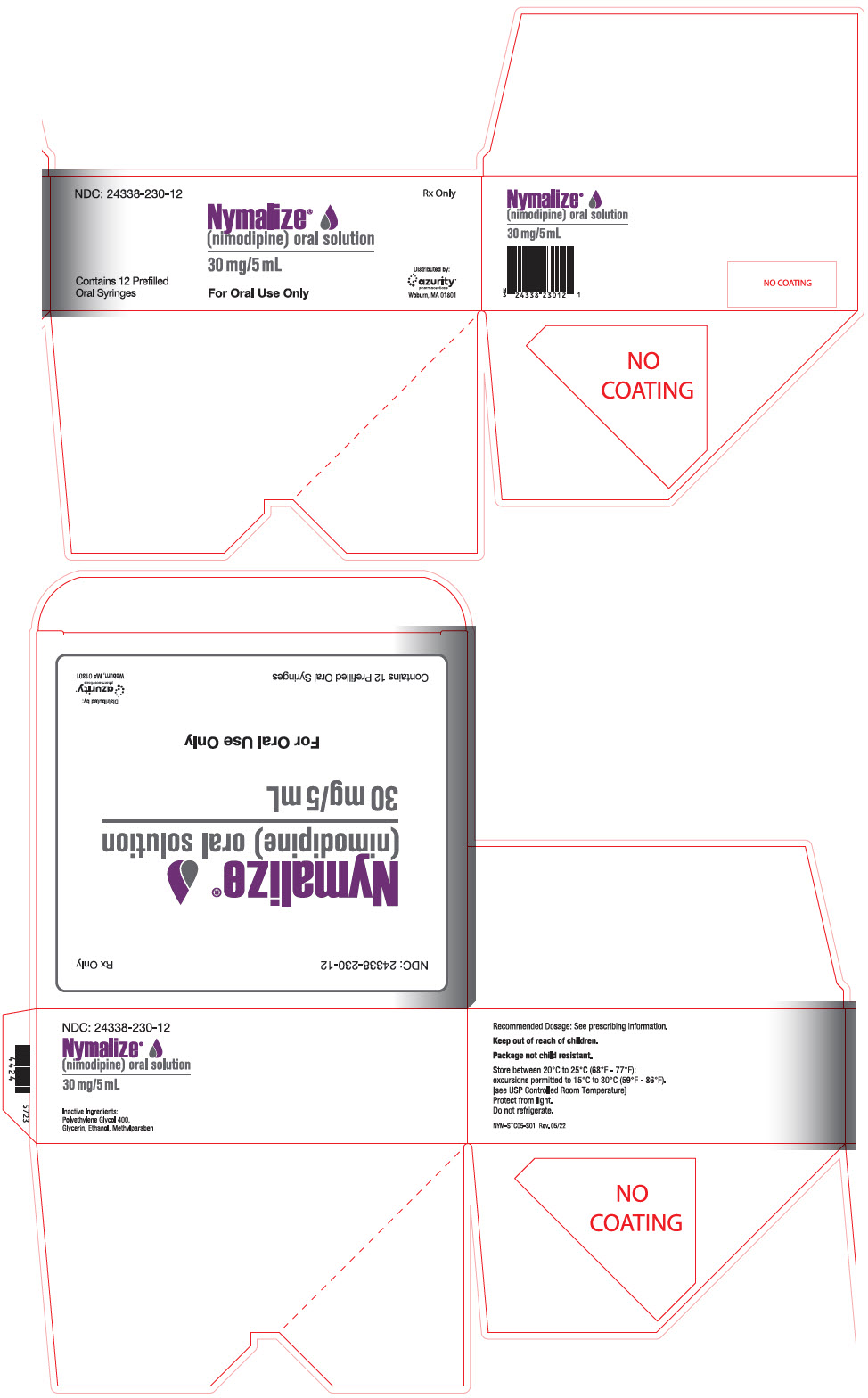
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 5 mL Syringe Package Carton
NDC: 24338-230-12
Rx Only
Nymalize®
(nimodipine) oral solution
30 mg/5 mL
Contains 12 Prefilled
Oral Syringes
For Oral Use Only
Distributed by:
azurity®
pharmaceuticals
Woburn, MA 01801
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Hypotension
Blood pressure should be carefully monitored during treatment with NYMALIZE. In clinical studies of patients with subarachnoid hemorrhage, about 5% of nimodipine-treated patients compared to 1% of placebo-treated patients had hypotension and about 1% of nimodipine-treated patients left the study because of this [see Adverse Reactions (6)].
5.2 Possible Increased Risk of Adverse Reactions in Patients with Cirrhosis
Given that the plasma levels of nimodipine are increased in patients with cirrhosis, these patients are at higher risk of adverse reactions. Therefore, monitor blood pressure and pulse rate closely and administer a lower dosage [see Dosage and Administration (2.4), Clinical Pharmacology (12.3)].
5.3 Possible Increased Risk of Hypotension with Strong CYP3A4 Inhibitors
Concomitant use of strong inhibitors of CYP3A4, such as some macrolide antibiotics (e.g., clarithromycin, telithromycin), some HIV protease inhibitors (e.g., indinavir, nelfinavir, ritonavir, saquinavir), some HCV protease inhibitors (e.g., boceprevir, telaprevir), some azole antimycotics (e.g., ketoconazole, itraconazole, posaconazole, voriconazole), conivaptan, delavirdine, and nefazodone with nimodipine should generally be avoided because of a risk of significant hypotension [see Drug Interactions (7.2)].
5.4 Possible Reduced Efficacy with Strong CYP3A4 Inducers
Concomitant use of strong CYP3A4 inducers (e.g., carbamazepine, phenobarbital, phenytoin, rifampin, St. John's wort) and nimodipine should generally be avoided, as nimodipine plasma concentration and efficacy may be significantly reduced [see Drug Interactions (7.3)].
- Hypotension: Monitor blood pressure. (5.1)
- Patients with Cirrhosis: Higher risk of adverse reactions. Monitor blood pressure and pulse. (5.2)
- CYP3A4 Strong Inhibitors: May significantly increase risk of hypotension. Concomitant use with NYMALIZE should generally be avoided. (5.3)
- CYP3A4 Strong Inducers: May significantly reduce efficacy of nimodipine. Concomitant use with NYMALIZE should generally be avoided. (5.4)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Blood Pressure Lowering Drugs
Nimodipine may increase the blood pressure lowering effect of concomitantly administered anti-hypertensives such as diuretics, beta-blockers, ACE inhibitors, angiotensin receptor blockers, other calcium channel blockers, α-adrenergic blockers, PDE5 inhibitors, and α-methyldopa. In Europe, nimodipine was observed to occasionally intensify the effect of antihypertensive drugs taken concomitantly by hypertensive patients; this phenomenon was not observed in North American clinical trials. Blood pressure should be carefully monitored, and dose adjustment of the blood pressure lowering drug(s) may be necessary.
7.2 CYP3A4 Inhibitors
Nimodipine plasma concentration can be significantly increased when concomitantly administered with strong CYP3A4 inhibitors. As a consequence, the blood pressure lowering effect may be increased. Therefore, the concomitant administration of NYMALIZE and strong CYP3A4 inhibitors should generally be avoided [see Warnings and Precautions (5.3)]. Strong CYP3A4 inhibitors include some members of the following classes:
- macrolide antibiotics (e.g., clarithromycin, telithromycin),
- HIV protease inhibitors (e.g., indinavir, nelfinavir, ritonavir, saquinavir),
- HCV protease inhibitors (e.g., boceprevir, telaprevir),
- azole antimycotics (e.g., ketoconazole, itraconazole, posaconazole, voriconazole),
- conivaptan, delavirdine, nefazodone
Nimodipine plasma concentration can also be increased in the presence of moderate and weak inhibitors of CYP3A4. If nimodipine is concomitantly administered with these drugs, blood pressure should be monitored, and a reduction of the nimodipine dose may be necessary. Moderate and weak CYP3A4 inhibitors include alprozalam, ameprenavir, amiodarone, aprepitant, atazanavir, cimetidine, cyclosporine, diltiazem, erythromycin, fluconazole, fluoxetine, isoniazid, oral contraceptives, quinuprestin/dalfopristin, valproic acid, and verapamil.
A study in eight healthy volunteers has shown a 50% increase in mean peak nimodipine plasma concentrations and a 90% increase in mean area under the curve, after a one-week course of cimetidine at 1,000 mg/day and nimodipine at 90 mg/day. This effect may be mediated by the known inhibition of hepatic cytochrome P-450 (CYP) by cimetidine, which could decrease first-pass metabolism of nimodipine.
Grapefruit juice inhibits CYP3A4. Ingestion of grapefruit/grapefruit juice is not recommended while taking nimodipine.
7.3 CYP3A4 Inducers
Nimodipine plasma concentration and efficacy may be significantly reduced when concomitantly administered with strong CYP3A4 inducers. Therefore, concomitant use of NYMALIZE with strong CYP3A4 inducers (e.g., carbamazepine, phenobarbital, phenytoin, rifampin, St. John's wort) should generally be avoided [see Warnings and Precautions (5.4)].
Moderate and weak inducers of CYP3A4 may also reduce the efficacy of nimodipine. Patients on these should be closely monitored for lack of effectiveness, and a nimodipine dosage increase may be required. Moderate and weak CYP3A4 inducers include, for example: amprenavir, aprepitant, armodafinil, bosentan, efavirnenz, etravirine, Echinacea, modafinil, nafcillin, pioglitazone, prednisone, rufinamide, and vemurafenib.
- Anti-Hypertensives: May increase risk of hypotension. Monitor blood pressure. (7.1)
- CYP3A4 Moderate and Weak Inhibitors: May increase risk of hypotension. Monitor blood pressure. Dose reduction of NYMALIZE may be needed. Avoid grapefruit juice. (7.2)
- CYP3A4 Moderate and Weak Inducers: May reduce efficacy of NYMALIZE. Dose increase may be needed. (7.3)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Administration Instructions
Administer only enterally (e.g., oral, nasogastric tube, or gastric tube route). Do not administer intravenously or by other parenteral routes. For all routes of administration, begin NYMALIZE within 96 hours of the onset of SAH. Administer one hour before a meal or two hours after a meal for all routes of administration [see Clinical Pharmacology (12.3)].
2.2 Administration by Oral Route
The recommended oral dosage is 10 mL (60 mg) every 4 hours for 21 consecutive days.
2.3 Administration Via Nasogastric or Gastric Tube
Using the supplied prefilled oral syringe labeled "For Oral Use Only", administer 10 mL (60 mg) every 4 hours into a nasogastric or gastric tube for 21 consecutive days. For each dose, refill the syringe with 10 mL of 0.9% saline solution and then flush any remaining contents from nasogastric or gastric tube into the stomach.
2.4 Dosage Adjustments in Patients with Cirrhosis
In patients with cirrhosis, reduce the dosage to 5 mL (30 mg) every 4 hours [see Warnings and Precautions (5.2), Clinical Pharmacology (12.3)].
- Administer only enterally (e.g., oral, nasogastric tube, or gastric tube route). Do not administer intravenously or by other parenteral routes. (2.1)
- Give one hour before a mealor two hours after a meal. (2.1)
- Start dosing within 96 hours of the SAH. (2.1)
- Recommended dose is 10 mL (60 mg) every 4 hours for 21 consecutive days. (2.2)
- Nasogastric or Gastric Tube Administration: Administer 10 mL (60 mg) every 4 hours with supplied prefilled oral syringe. Refill syringe with 10 mL of 0.9% saline water solution; flush remaining contents from nasogastric or gastric tube into stomach. (2.3)
- Patients with Cirrhosis: Reduce dosage to 5 mL (30 mg) every 4 hours. (2.4)
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a two-year study in rats, the incidences of adenocarcinoma of the uterus and Leydig cell adenoma of the testes were increased at 1800 ppm nimodipine in the diet (approximately 90-120 mg/kg/day). The increases were not statistically significant, however, and the higher rates were within the historical control range for these tumors. Nimodipine was found not to be carcinogenic in a 91-week mouse study, but the high dose of 1800 ppm nimodipine in the diet (approximately 550-775 mg/kg/day) was associated with an increased mortality rate.
Mutagenesis
Mutagenicity studies, including the Ames, micronucleus, and dominant lethal assays, were negative.
Impairment of Fertility
Nimodipine did not impair the fertility and general reproductive performance of male and female rats following oral doses of up to 30 mg/kg/day when administered prior to mating and continuing in females to day 7 of pregnancy. This dose in a rat is similar to a clinical dose of 60 mg every 4 hours in a 60 kg patient, on a body surface area (mg/m2) basis.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
The safety and efficacy of NYMALIZE (nimodipine oral solution) in the treatment of patients with SAH is based on adequate and well-controlled studies of nimodipine oral capsules in patients with SAH. NYMALIZE (nimodipine oral solution) has comparable bioavailability to nimodipine oral capsules.
Nimodipine has been shown in 4 randomized, double-blind, placebo-controlled trials to reduce the severity of neurological deficits resulting from vasospasm in patients who have had a recent SAH (Studies 1, 2, 3, and 4).
The trials used doses ranging from 20-30 mg to 90 mg every 4 hours, with drug given for 21 days in 3 studies, and for at least 18 days in the other. Three of the four trials followed patients for 3-6 months. Three of the trials studied relatively well patients, with all or most patients in Hunt and Hess Grades I - III (essentially free of focal deficits after the initial bleed). Study 4 studied much sicker patients with Hunt and Hess Grades III - V. Studies 1 and 2 were similar in design, with relatively unimpaired SAH patients randomized to nimodipine or placebo. In each, a judgment was made as to whether any late-developing deficit was due to spasm or other causes, and the deficits were graded. Both studies showed significantly fewer severe deficits due to spasm in the nimodipine group; Study 2 showed fewer spasm- related deficits of all severities. No effect was seen on deficits not related to spasm. See Table 2.
Table 2: Deficits in Patients with Hunt and Hess Grades I to III in Study 1 and Study 2|
Study |
Grade* |
Treatment |
Patients | ||
|---|---|---|---|---|---|
|
Number Analyzed |
Number of Patients with Any Deficit Due to Spasm |
Numbers with Severe Deficit | |||
| |||||
|
Study 1 |
I-III |
Nimodipine 20-30 mg every 4 hours |
56 |
13 |
1 |
|
Placebo |
60 |
16 |
8† | ||
|
Study 2 |
I-III |
Nimodipine 60 mg every 4 hours |
31 |
4 |
2 |
|
Placebo |
39 |
11 |
10† |
Study 3 was a 554-patient trial that included SAH patients with all grades of severity (89% were in Hunt and Hess Grades I-III). In Study 3, patients were treated with placebo or 60 mg of nimodipine every 4 hours. Outcomes were not defined as spasm related or not but there was a significant reduction in the overall rate of brain infarction and severely disabling neurological outcome at 3 months (Table 3):
Table 3: Degree of Recovery or Disability in Study 3 (89% Hunt and Hess Grades I-III)|
Nimodipine |
Placebo | |
|---|---|---|
| ||
|
Total patients |
278 |
276 |
|
Good recovery |
199* |
169 |
|
Moderate disability |
24 |
16 |
|
Severe disability |
12† |
31 |
|
Death |
43‡ |
60 |
Study 4 enrolled much sicker patients, (Hunt and Hess Grades III-V), who had a high rate of death and disability, and used a dose of 90 mg every 4 hours, but was otherwise similar to Study 1 and Study 2. Analysis of delayed ischemic deficits, many of which result from spasm, showed a significant reduction in spasm-related deficits. Among analyzed patients (72 nimodipine, 82 placebo), there were the following outcomes (Table 4).
Table 4: Neurological Ischemic Deficits in Study 4 [Hunt and Hess Grades III-V]|
Delayed Ischemic Deficits (DID) |
Permanent Deficits | |||
|---|---|---|---|---|
|
Nimodipine 90 mg every 4 hours |
Placebo |
Nimodipine 90 mg every 4 hours |
Placebo | |
|
n (%) |
n (%) |
n (%) |
n (%) | |
| ||||
|
DID Spasm Alone |
8 (11)* |
25 (31) |
5 (7)* |
22 (27) |
|
DID Spasm Contributing |
18 (25) |
21 (26) |
16 (22) |
17 (21) |
|
DID Without Spasm |
7 (10) |
8 (10) |
6 (8) |
7 (9) |
|
No DID |
39 (54) |
28 (34) |
45 (63) |
36 (44) |
When data were combined for Study 3 and Study 4, the treatment difference on success rate (i.e., good recovery) on the Glasgow Outcome Scale was 25.3% (nimodipine) versus 10.9% (placebo) for Hunt and Hess Grades IV or V. Table 5 demonstrates that nimodipine tends to improve good recovery of SAH patients with poor neurological status post-ictus, while decreasing the numbers with severe disability and vegetative survival.
Table 5: Glasgow Outcome Scale in Combined Studies 3 and 4|
Glasgow Outcome* |
Nimodipine (n=87) |
Placebo |
|---|---|---|
| ||
|
Good Recovery |
22 (25.3%) |
11 (10.9%) |
|
Moderate Disability |
8 (9.2%) |
12 (11.9%) |
|
Severe Disability |
6 (6.9%) |
15 (14.9%) |
|
Vegetative Survival |
4 (4.6%) |
9 (8.9%) |
|
Death |
47 (54.0%) |
54 (53.5%) |
A dose-ranging study comparing 30 mg, 60 mg, and 90 mg doses found a generally low rate of spasm-related neurological deficits but no dose response relationship.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
NYMALIZE (nimodipine) Oral Solution 6 mg/mL is a pale yellow solution and is supplied as follows:
-
NDC 24338-260-08: 8 oz. bottle (237 mL) 60 mg/10 mL (6 mg/mL)
-
NDC 24338-260-12: Carton containing 12 individually wrapped 10 mL packages.
Each package contains one 60 mg/10 mL unit-dose prefilled oral syringe with a purple plunger (NDC 24338-260-10). -
NDC 24338-230-12: Carton containing 12 individually wrapped 5 mL packages.
Each package contains one 30 mg/5 mL unit-dose prefilled oral syringe with a white plunger (NDC 24338-230-05).
Store between 20ºC to 25ºC (68ºF to 77ºF); excursions permitted to 15ºC to 30ºC (59ºF to 86ºF) [see USP Controlled Room Temperature].
Protect from light.
Do not refrigerate.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Inform patients that the most frequent adverse reaction associated with nimodipine is decreased blood pressure [see Warnings and Precautions (5.1)]. Inform them that use of NYMALIZE with anti-hypertensives can cause increased drop in blood pressure [see Drug Interactions (7.1)].
Patients should be aware that ingestion of grapefruit or grapefruit juice should be avoided when taking NYMALIZE due to its ability to increase nimodipine plasma concentrations and potential to increase the risk of hypotension [see Drug Interactions (7.2)].
Advise patients to notify their healthcare provider if they become pregnant during treatment or plan to become pregnant during therapy [see Use in Specific Populations (8.1)].
Advise female patients to notify their physicians if they intend to breastfeed or are breastfeeding an infant [see Use in Specific Populations (8.2)].
