HEALSURE SKIN PROTECTANT
84010-122
3602a8f1-3589-5793-e063-6294a90adbe4
HUMAN OTC DRUG LABEL
May 25, 2025
Jiangxi Hemei Pharmaceutical Co., Ltd
DUNS: 724892056
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Dimethicone 2% SKIN PROTECTANT
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
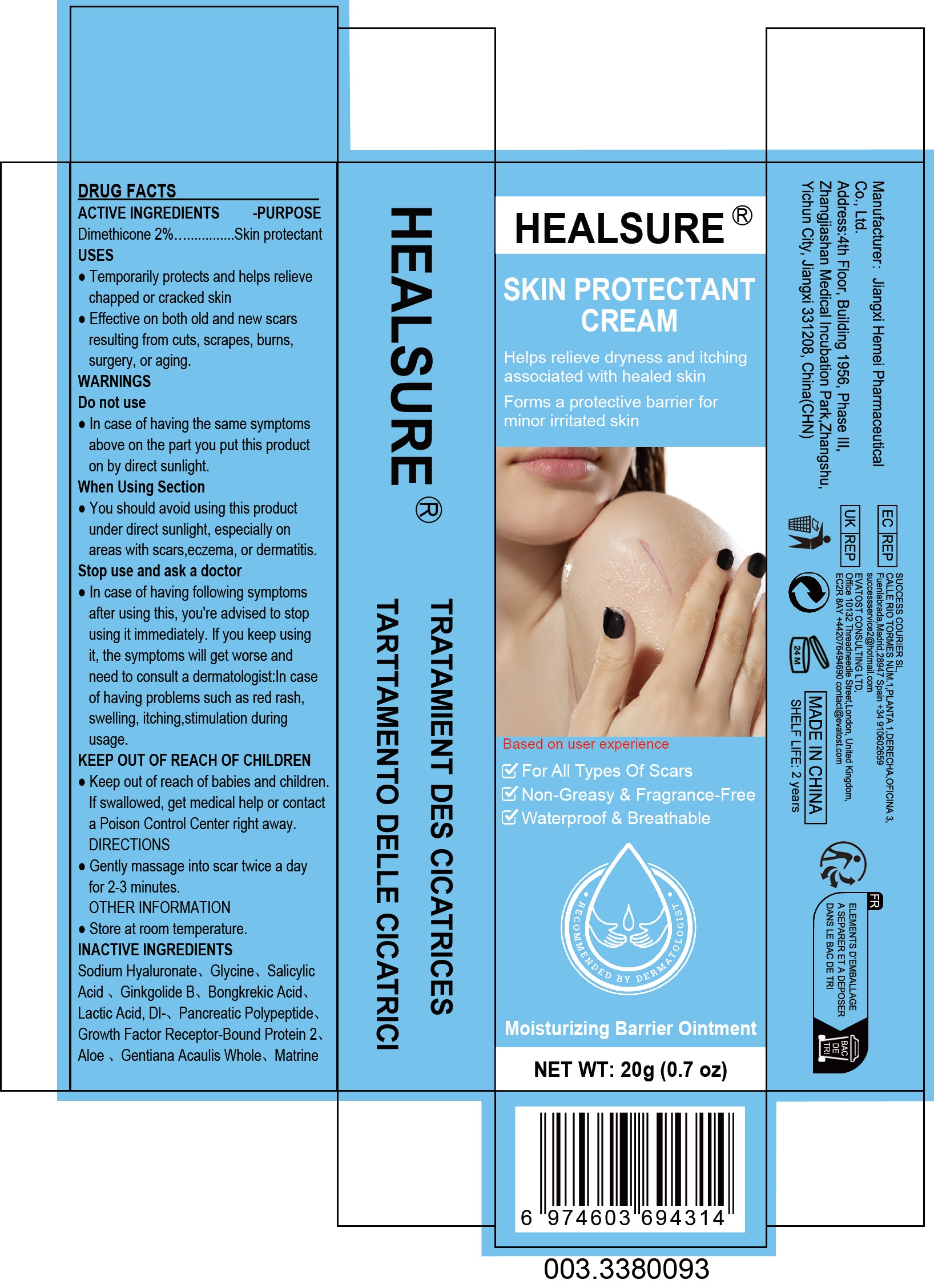
INDICATIONS & USAGE SECTION
Use
·Temporarily protects and helps relieve chapped or cracked skin
·Effective on both old and new scars resulting from cuts, scrapes, burns,surgery, or aging.
OTC - STOP USE SECTION
Stop Use
·In case of having following symptoms after using this,you're advised to stop using it immediately. If you keep using it,the symptoms will get worse and need to consult a dermatologist:In case of having problems such as red rash,swelling, itching,stimulation during usage.
OTC - ACTIVE INGREDIENT SECTION
Active Ingredient
Dimethicone 2%
OTC - PURPOSE SECTION
Purpose
Skin protectant
WARNINGS SECTION
Warnings
·In case of having the same symptoms above on the part you put this product on by direct sunlight.
·You should avoid using this product under direct sunlight,especially on areas with scars, eczema, or dermatitis. ·In case of having following symptoms after using this,you're advised to stop using it immediately. If you keep using it,the symptoms will get worse and need to consult a dermatologist:In case of having problems such as red rash,swelling, itching,stimulation during usage.
OTC - DO NOT USE SECTION
Do not use
·In case of having the same symptoms above on the part you put this product on by direct sunlight.
OTC - WHEN USING SECTION
When Using
·You should avoid using this product under direct sunlight,especially on areas with scars, eczema, or dermatitis.
OTC - ASK DOCTOR SECTION
Ask Doctor
·In case of having following symptoms after using this,you're advised to stop using it immediately. If you keep using it,the symptoms will get worse and need to consult a dermatologist:In case of having problems such as red rash,swelling, itching,stimulation during usage.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep Out Of Reach Of Children
·Keep out of reach of babies and children, If swallowed, get medical help or contact a Poison Control Cente right away.
DOSAGE & ADMINISTRATION SECTION
Directions
·Gently massage into scar twice a day for 2-3 minutes
STORAGE AND HANDLING SECTION
Other information
Store at room temperature.
INACTIVE INGREDIENT SECTION
Inactive ingredients
Sodium Hyaluronate 、Glycine 、Salicylic Acid 、Ginkgolide B、Bongkrekic Acid 、Lactic Acid,Dl-、Pancreatic Polypeptide Growth Factor Receptor-Bound Protein 2 、Aloe、GentianaAcaulis Whole 、Matrine
