Polymyxin B Sulfate and Trimethoprim
Polymyxin B Sulfate andTrimethoprim Ophthalmic Solution, USP (Sterile)
31a7ad1f-3f58-41a0-94f0-36674285bb61
HUMAN PRESCRIPTION DRUG LABEL
Sep 1, 2023
Preferred Pharmaceuticals Inc.
DUNS: 791119022
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Polymyxin B Sulfate and Trimethoprim Sulfate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
Polymyxin B sulfate and trimethoprim ophthalmic solution, USP is a sterile antimicrobial solution for topical ophthalmic use. It has pH of 4.0 to 5.5 and osmolality of 270 to 310 mOsm/kg.
Chemical Names:
Trimethoprim sulfate, 2,4-diamino-5-(3,4,5-trimethoxybenzyl) pyrimidine
sulfate, is a white, odorless, crystalline powder with a molecular weight of
678.72 and the following structural formula:
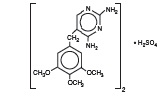
C28H38N8O10S Mol. Wt. 678.72
Polymyxin B sulfate is the sulfate salt of polymyxin B1 and B2 which are produced by the growth of Bacillus polymyxa (Prazmowski) Migula (Fam. Bacillaceae). It has a potency of not less than 6,000 polymyxin B units per mg, calculated on an anhydrous basis. The structural formula is:
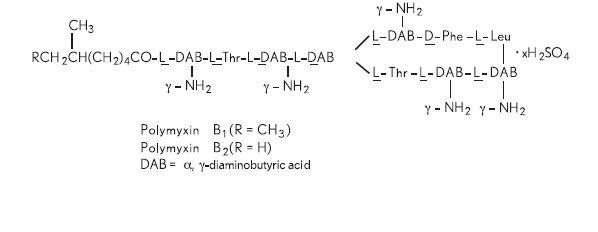
Each mL contains: Actives: polymyxin B sulfate equal to 10,000 polymyxin B units, trimethoprim sulfate (equivalent to trimethoprim 1 mg); Inactives: purified water, sodium chloride. Sulfuric acid and, if necessary, sodium hydroxide may be added to adjust pH (4.0 – 5.5). Preservative: benzalkonium chloride 0.004%
HOW SUPPLIED SECTION
HOW SUPPLIED
Polymyxin B sulfate and trimethoprim ophthalmic solution, USP* containing 10,000 polymyxin B units and 1 mg trimethoprim per mL, is supplied in a plastic bottle with a controlled drop tip and a natural cap in the following size:
NDC 68788-8152-1 10 mL
|
DO NOT USE IF IMPRINTED NECKBAND IS NOT INTACT. |
Storage:
Store at 15°C to 25°C (59°F to 77°F). PROTECT FROM LIGHT.
*Does not meet USP packaging specification for light resistance.
RETAIN IN CARTON UNTIL TIME OF USE.
Distributed by:
Bausch & Lomb Americas Inc.
Bridgewater, NJ 08807 USA
Manufactured by:
Bausch & Lomb Incorporated
Tampa, FL 33637 USA
© 2023 Bausch & Lomb Incorporated or its affiliates
Relabeled By: Preferred Pharmaceuticals Inc.
Revised: June 2023
9117804**(Folded)**
9117904**(Flat)**
