Thinnr BOOST
Thinnr BOOST
2bda8ba9-8626-aafd-e063-6294a90adbe8
HUMAN OTC DRUG LABEL
Aug 20, 2025
SLIMCELL, LLC
DUNS: 097997221
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Adeps suillus, Andrenalinum, Adrenocorticotrophin, Calcarea carbonica, Chelidonium, Pituitarum, Thyroidinum
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
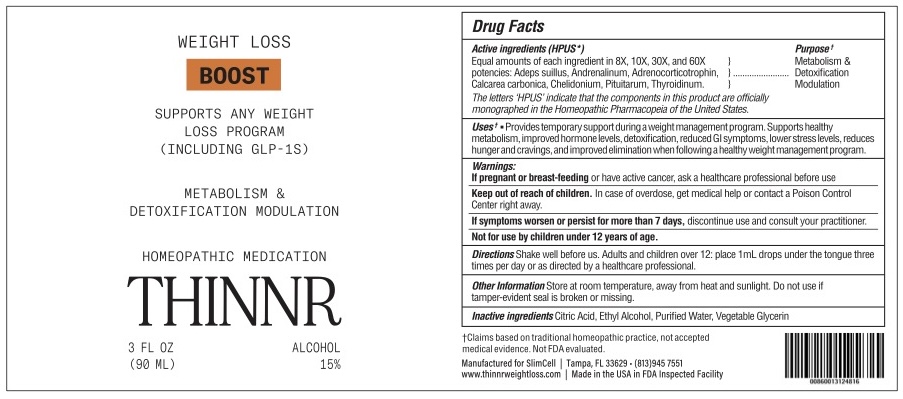

INDICATIONS & USAGE SECTION
Uses*:
Uses*: Provides temporary support during a weight management program. Supports healthy metabolism, improved hormone levels, detoxification, lower stress levels, reduces hunger and cravings, and improved elimination when following a healthy weight management program.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
OTC - PREGNANCY OR BREAST FEEDING SECTION
If pregnant or breast-feeding
If pregnant or breast-feeding or have active cancer, ask a healthcare professional before use
OTC - PURPOSE SECTION
Metabolism and detoxification modulation
OTHER SAFETY INFORMATION
Other Information
Store at room temperature, away from heat and sunlight.
Do not use if tamper-evident seal is broken or missing.
INACTIVE INGREDIENT SECTION
Inactive Ingredients
Inactive Ingredients: Ethyl Alcohol, Glycerin, Purified Water.
DOSAGE & ADMINISTRATION SECTION
Directions
Shake well before us. Adults and children over 12: place 1mL drops under the tongue three times per day or as directed by a healthcare professional.
OTC - ACTIVE INGREDIENT SECTION
HPUS Active Ingredients
HPUS Active Ingredients: Equal amounts of each ingredient in 8X, 10X, 30X, and 60X potencies: Adeps suillus, Andrenalinum, Adrenocorticotrophin, Calcarea carbonica, Chelidonium, Pituitarum, and Thyroidinum.
WARNINGS SECTION
Warnings
Warnings: If symptoms worsen or persist for more than 7 days, discontinue use and consult your practitioner.
