Lidocaine Hydrochloride
b54f5334-cdf1-8a22-e053-2995a90ad6da
HUMAN PRESCRIPTION DRUG LABEL
Jul 14, 2023
Afaxys Pharma LLC
DUNS: 080508656
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
lidocaine hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
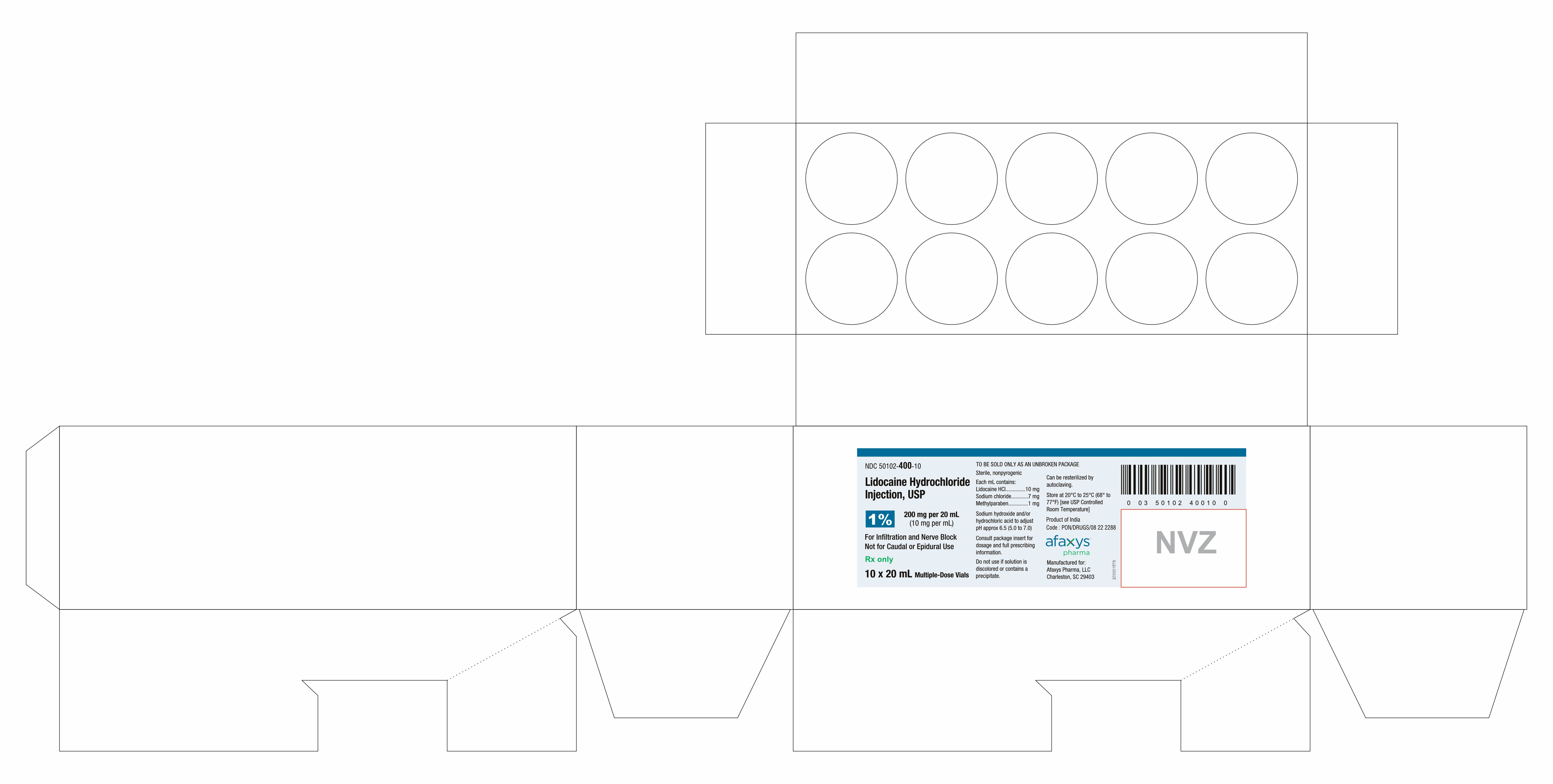
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 10 Vial Tray Label
NDC 50102-400-10
Lidocaine Hydrochloride
Injection, USP
1%
200 mg per 20 mL
(10 mg per mL)
For Infiltration and Nerve Block
Not for Caudal or Epidural Use
Rx only
10 x 20 mL Multiple-Dose Vials
OVERDOSAGE SECTION
OVERDOSAGE:
Acute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use of local anesthetics or to unintended subarachnoid injection of local anesthetic solution (seeADVERSE REACTIONS****,WARNINGS, and****PRECAUTIONS).
Management of Local Anesthetic Emergencies
The first consideration is prevention, best accomplished by careful and constant monitoring of cardiovascular and respiratory vital signs and the patient's state of consciousness after each local anesthetic injection. At the first sign of change, oxygen should be administered.
The first step in the management of convulsions, as well as underventilation or apnea due to unintended subarachnoid injection of drug solution, consists of immediate attention to the maintenance of a patent airway and assisted or controlled ventilation with oxygen and a delivery system capable of permitting immediate positive airway pressure by mask. Immediately after the institution of these ventilatory measures, the adequacy of the circulation should be evaluated, keeping in mind that drugs used to treat convulsions sometimes depress the circulation when administered intravenously. Should convulsions persist despite adequate respiratory support, and if the status of the circulation permits, small increments of an ultra-short acting barbiturate (such as thiopental or thiamylal) or a benzodiazepine (such as diazepam) may be administered intravenously. The clinician should be familiar, prior to the use of local anesthetics, with these anticonvulsant drugs. Supportive treatment of circulatory depression may require administration of intravenous fluids and, when appropriate, a vasopressor as directed by the clinical situation (e.g., ephedrine).
If not treated immediately, both convulsions and cardiovascular depression can result in hypoxia, acidosis, bradycardia, arrhythmias and cardiac arrest. Underventilation or apnea due to unintentional subarachnoid injection of local anesthetic solution may produce these same signs and also lead to cardiac arrest if ventilatory support is not instituted. If cardiac arrest should occur, standard cardiopulmonary resuscitative measures should be instituted.
Endotracheal intubation, employing drugs and techniques familiar to the clinician, may be indicated, after initial administration of oxygen by mask, if difficulty is encountered in the maintenance of a patent airway or if prolonged ventilatory support (assisted or controlled) is indicated.
Dialysis is of negligible value in the treatment of acute overdosage with lidocaine HCl.
The oral LD50 of lidocaine HCl in non-fasted female rats is 459 (346 to 773) mg/kg (as the salt) and 214 (159 to 324) mg/kg (as the salt) in fasted female rats.
HOW SUPPLIED SECTION
HOW SUPPLIED:
Lidocaine Hydrochloride Injection USP, 1% is provided as a clear colourless solution filled in 20 mL clear moulded glass vial USP Type I with 20 mm Chlorobutyl rubber stopper and 20 mm aluminium violet flip-off seal.
LIDOCAINE HYDROCHLORIDE INJECTION USP, 1 % is supplied as follows:
|
Product Code |
Unit of Sale |
Strength |
Each |
|
400 |
NDC 50102-400-10 |
1% (200 mg per 20 mL) |
20 mL Multiple Dose Vial |
Store at 20° to 25° C (68° to 77° F) [see USP Controlled Room Temperature].
Protect from light.
To report SUSPECTED ADVERSE REACTIONS, contact Afaxys at 1-855-888-2467 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Manufactured for
Afaxys Pharma, LLC
Charleston, SC 29403
www.afaxys.com
Revised: June 2022
