YONHISDAT Sunscreen Powder
3aeb0f66-d613-9bc8-e063-6294a90a691d
HUMAN OTC DRUG LABEL
Aug 1, 2025
PANERGYTEK USA INC
DUNS: 122142088
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
titanium dioxide, zinc oxide powder
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (16)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
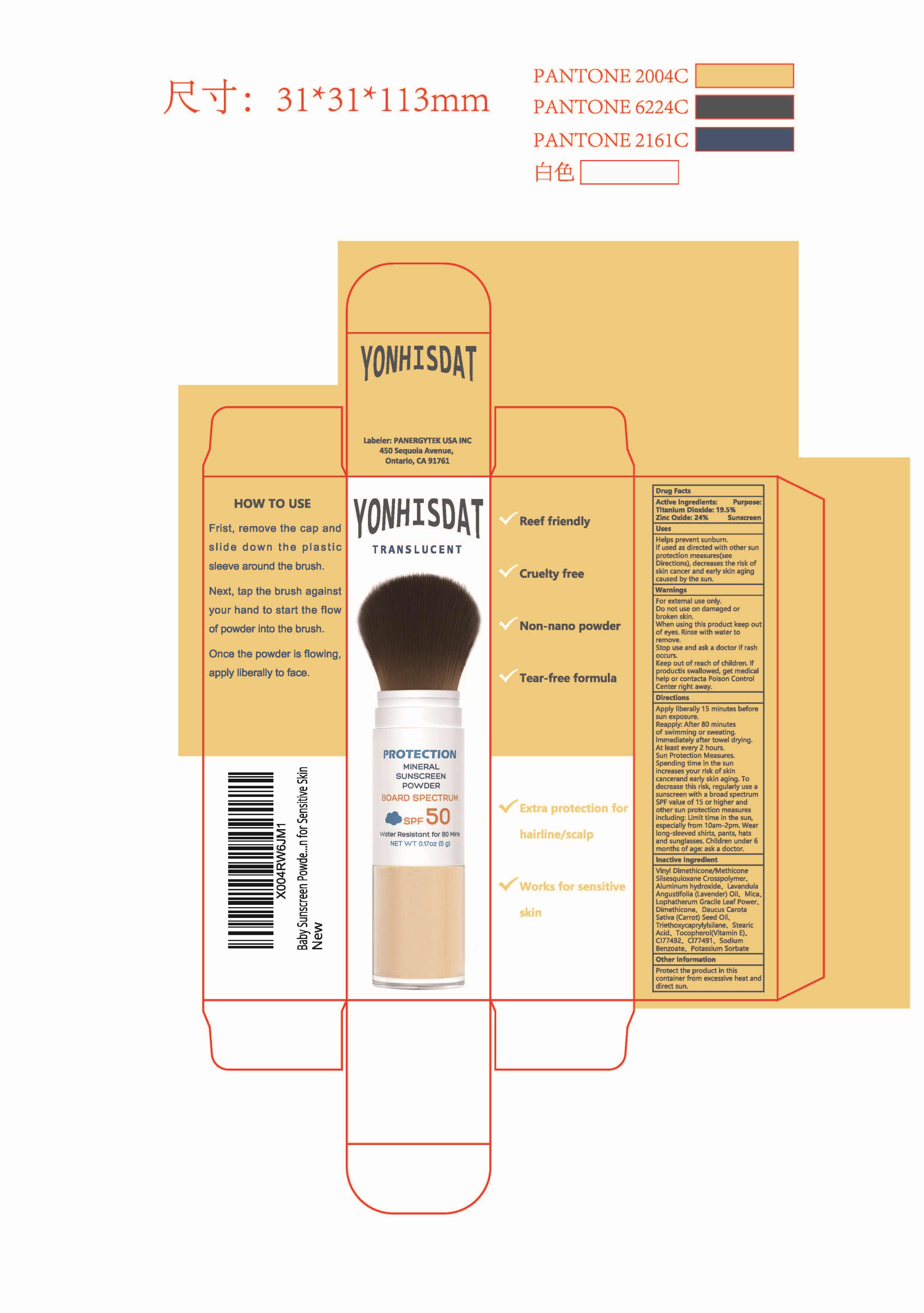
INDICATIONS & USAGE SECTION
Apoly liberally i5 mnutes before sunexoosure.pants, hats andsunglasses.
Children under 6months of age: ask a doctor
Reapph: Aler 80 minues oswinming or sneaing immetlaletater ionel dning Atleast
eveny2hours.Sun precion leasures Spending ime in the sun increasesyour isk
oifshincancer and eaty skinaging T
decrease this rsk requlanuse a sunsren uith a bradspecnum spf value of 15 or
higtherAnd ohersun prolecion measureshclud ng imtime ihthe sun especdaly fom
10am.2am.
OTC - ACTIVE INGREDIENT SECTION
TITANIUM DIOXIDE
19.5%
ZINC OXIDE24%
OTC - PURPOSE SECTION
Sunscreen
OTC - WHEN USING SECTION
Helps prevent sunbum fused as directed with other sunprotection measures(see Directions)decreases the risk of skin cancer andeary skin aging caused by the sun
DOSAGE & ADMINISTRATION SECTION
Apoly liberally i5 mnutes before sunexoosure.pants, hats andsunglasses.
Children under 6months of age: ask a doctor
Reapph: Aler 80 minues oswinming or sneaing immetlaletater ionel dning Atleast
eveny2hours.Sun precion leasures Spending ime in the sun increasesyour isk
oifshincancer and eaty skinaging T
decrease this rsk requlanuse a sunsren uith a bradspecnum spf value of 15 or
higtherAnd ohersun prolecion measureshclud ng imtime ihthe sun especdaly fom
10am.2am.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children. lf swallowed, get medical help or contact a Poison Control Center right away
OTHER SAFETY INFORMATION
Protect this product in this container from excessive heat and direct sun.
INACTIVE INGREDIENT SECTION
Vinyl Dimethicone/Methicone Silsesquioxane Crosspolymer
Aluminum hydroxide
Mica
Lophatherum Gracile Leaf
Dimethicone
Triethoxycaprylylsilane
Stearic Acid
Tocopherol
CI 77492
CI 77491
Sodium Benzoate
Potassium Sorbate
Daucus Carota Sativa (Carrot) Seed Oil
Lavandula Angustifolia (Lavender) Oil
WARNINGS SECTION
For enemal use onyDo nd use on damaged or icken shnwmen using his poductkep ou
oeyes Rise wth walerto remove slop use and ask adoco lrash occursKeep out d
reachof thilien. producs
swallowed. get medical helpor contact a Potson Control Center rightaway
