Enoxaparin Sodium
These highlights do not include all the information needed to use ENOXAPARIN SODIUM INJECTION safely and effectively. See full prescribing information forENOXAPARIN SODIUM INJECTION.ENOXAPARIN SODIUM Injection, for subcutaneous and intravenous use Initial U.S. Approval: 1993
3e6bf2b3-9339-4104-bda3-ad86a1949703
HUMAN PRESCRIPTION DRUG LABEL
Jul 1, 2023
Amphastar Pharmaceuticals, Inc.
DUNS: 024736733
Products 8
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Enoxaparin sodium
PRODUCT DETAILS
INGREDIENTS (2)
enoxaparin sodium
PRODUCT DETAILS
INGREDIENTS (2)
Enoxaparin sodium
PRODUCT DETAILS
INGREDIENTS (2)
Enoxaparin sodium
PRODUCT DETAILS
INGREDIENTS (2)
Enoxaparin sodium
PRODUCT DETAILS
INGREDIENTS (2)
Enoxaparin sodium
PRODUCT DETAILS
INGREDIENTS (2)
Enoxaparin sodium
PRODUCT DETAILS
INGREDIENTS (2)
Enoxaparin sodium
PRODUCT DETAILS
INGREDIENTS (2)
Drug Labeling Information
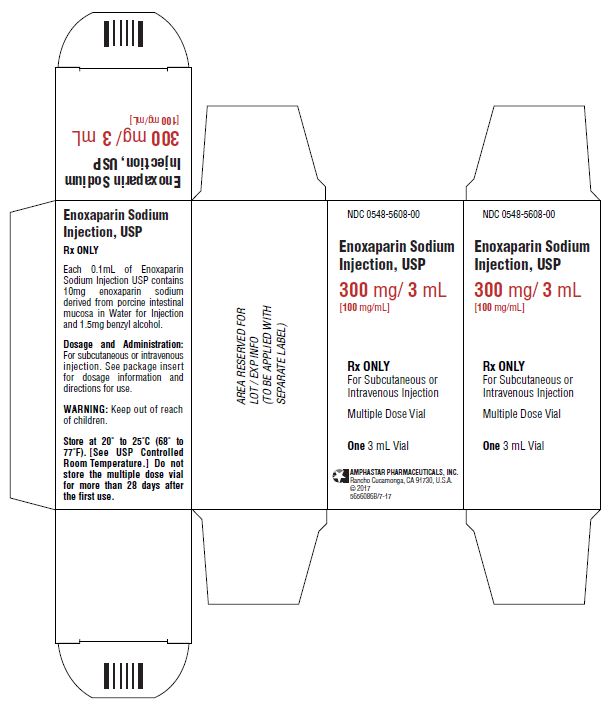
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL: Multiple-Dose Vial Carton
NDC 0548-5608-00
Enoxaparin Sodium
Injection, USP
300 mg/3 mL
[100 mg/mL]
Rx ONLY
For Subcutaneous or
Intravenous Injection
Multiple Dose Vial
One 3 mL Vial
AMPHASTAR PHARMACEUTICALS, INC.
Rancho Cucamonga, CA 91730, U.S.A.
© 2017
5656086B/7-17
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
1.1 Prophylaxis of Deep Vein Thrombosis
Enoxaparin Sodium Injection is indicated for the prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE):
- in patients undergoing abdominal surgery who are at risk for thromboembolic complications [see Clinical Studies (14.1)]
- in patients undergoing hip replacement surgery, during and following hospitalization
- in patients undergoing knee replacement surgery
- in medical patients who are at risk for thromboembolic complications due to severely restricted mobility during acute illness
1.2 Treatment of Acute Deep Vein Thrombosis
Enoxaparin Sodium Injection is indicated for:
- the inpatient treatment of acute deep vein thrombosis with or without pulmonary embolism, when administered in conjunction with warfarin sodium
- the outpatient treatment of acute deep vein thrombosis without pulmonary embolism when administered in conjunction with warfarin sodium
1.3 Prophylaxis of Ischemic Complications of Unstable Angina and Non-Q-Wave
Myocardial Infarction
Enoxaparin Sodium Injection is indicated for the prophylaxis of ischemic complications of unstable angina and non-Q-wave myocardial infarction, when concurrently administered with aspirin.
1.4 Treatment of Acute ST-Segment Elevation Myocardial Infarction
Enoxaparin Sodium Injection, when administered concurrently with aspirin, has been shown to reduce the rate of the combined endpoint of recurrent myocardial infarction or death in patients with acute ST-segment elevation myocardial infarction (STEMI) receiving thrombolysis and being managed medically or with percutaneous coronary intervention (PCI).
Enoxaparin Sodium Injection is a low molecular weight heparin (LMWH) indicated for:
- Prophylaxis of deep vein thrombosis (DVT) in abdominal surgery, hip replacement surgery, knee replacement surgery, or medical patients with severely restricted mobility during acute illness (1.1)
- Inpatient treatment of acute DVT with or without pulmonary embolism (1.2)
- Outpatient treatment of acute DVT without pulmonary embolism (1.2)
- Prophylaxis of ischemic complications of unstable angina and non-Q-wave myocardial infarction (MI) (1.3)
- Treatment of acute ST-segment elevation myocardial infarction (STEMI) managed medically or with subsequent percutaneous coronary intervention (PCI) (1.4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Increased Risk of Hemorrhage
Cases of epidural or spinal hemorrhage and subsequent hematomas have been
reported with the use of Enoxaparin Sodium Injection and epidural or spinal
anesthesia/analgesia or spinal puncture procedures, resulting in long-term or
permanent paralysis. The risk of these events is higher with the use of
postoperative indwelling epidural catheters, with the concomitant use of
additional drugs affecting hemostasis such as NSAIDs, with traumatic or
repeated epidural or spinal puncture, or in patients with a history of spinal
surgery or spinal deformity [see Boxed Warning, Adverse Reactions (6.2) and Drug Interactions (7)].
To reduce the potential risk of bleeding associated with the concurrent use of
Enoxaparin Sodium Injection and epidural or spinal anesthesia/analgesia or
spinal puncture, consider the pharmacokinetic profile of Enoxaparin Sodium
Injection [see Clinical Pharmacology (12.3)].
Placement or removal of an epidural catheter or lumbar puncture is best
performed when the anticoagulant effect of Enoxaparin Sodium Injection is low;
however, the exact timing to reach a sufficiently low anticoagulant effect in
each patient is not known.
Placement or removal of a catheter should be delayed for at least 12 hours
after administration of lower doses (30 mg once or twice-daily or 40 mg once
daily) of Enoxaparin Sodium Injection, and at least 24 hours after the
administration of higher doses (0.75 mg/kg twice daily, 1 mg/kg twice daily,
or 1.5 mg/kg once daily) of Enoxaparin Sodium Injection.
Anti-Xa levels are still detectable at these time points, and these delays are
not a guarantee that neuraxial hematoma will be avoided. Patients receiving
the 0.75 mg/kg twice-daily dose or the 1 mg/kg twice daily dose should not
receive the second Enoxaparin Sodium Injection dose in the twice-daily regimen
to allow a longer delay before catheter placement or removal.
Likewise, although a specific recommendation for timing of a subsequent
Enoxaparin Sodium Injection dose after catheter removal cannot be made,
consider delaying this next dose for at least four hours, based on a benefit-
risk assessment considering both the risk for thrombosis and the risk for
bleeding in the context of the procedure and patient risk factors. For
patients with creatinine clearance <30mL/minute, additional considerations are
necessary because elimination of Enoxaparin Sodium Injection is more
prolonged; consider doubling the timing of removal of a catheter, at least 24
hours for the lower prescribed dose of Enoxaparin Sodium Injection (30 mg once
daily) and at least 48 hours for the higher dose (1 mg/kg/day) [see Clinical Pharmacology (12.3)].
Should the physician decide to administer anticoagulation in the context of
epidural or spinal anesthesia/analgesia or lumbar puncture, frequent
monitoring must be exercised to detect any signs and symptoms of neurological
impairment such as midline back pain, sensory and
motor deficits (numbness or weakness in lower limbs), and bowel and/or bladder
dysfunction.
Instruct patients to report immediately if they experience any of the above
signs or symptoms.
If signs or symptoms of spinal hematoma are suspected, initiate urgent
diagnosis and treatment including consideration for spinal cord decompression
even though such treatment may not prevent or reverse neurological sequelae.
Use Enoxaparin Sodium Injection with extreme caution in conditions with
increased risk of hemorrhage, such as bacterial endocarditis, congenital or
acquired bleeding disorders, active ulcerative and angiodysplastic
gastrointestinal disease, hemorrhagic stroke, or shortly after brain, spinal,
or ophthalmological surgery, or in patients treated concomitantly with
platelet
inhibitors.
Major hemorrhages including retroperitoneal and intracranial bleeding have
been reported.
Some of these cases have been fatal.
Bleeding can occur at any site during therapy with Enoxaparin Sodium
Injection. An unexplained fall in hematocrit or blood pressure should lead to
a search for a bleeding site.
5.2 Increased Risk of Bleeding following Percutaneous Coronary
Revascularization Procedures
To minimize the risk of bleeding following the vascular instrumentation during
the treatment of unstable angina, non-Q-wave myocardial infarction, and acute
ST-segment elevation myocardial infarction, adhere precisely to the intervals
recommended between Enoxaparin
Sodium Injection doses. It is important to achieve hemostasis at the puncture
site after PCI.In case a closure device is used, the sheath can be removed
immediately. If a manual compression method is used, sheath should be removed
6 hours after the last intravenous/ subcutaneous Enoxaparin Sodium Injection.
If the treatment with enoxaparin sodium is to be continued, the next scheduled
dose should be given no sooner than 6 to 8 hours after sheath removal. The
site of the procedure should be observed for signs of bleeding or hematoma
formation [see Dosage and Administration (2.1)].
5.3 Increased Risk of Bleeding in Patients with Concomitant Medical
Conditions
Enoxaparin Sodium Injection should be used with care in patients with a bleeding diathesis, uncontrolled arterial hypertension or a history of recent gastrointestinal ulceration, diabetic retinopathy, renal dysfunction and hemorrhage.
5.4 Risk of Heparin-Induced Thrombocytopenia with or without Thrombosis
Enoxaparin Sodium Injection may cause heparin-induced thrombocytopenia (HIT)
or heparininduced thrombocytopenia with thrombosis (HITTS). HITTS may lead to
organ infarction, limb ischemia, or death. Monitor thrombocytopenia of any
degree closely. Use of Enoxaparin Sodium Injection in patients with a history
of immune-mediated HIT within the past 100 days or in the presence of
circulating antibodies is contraindicated [see Contraindications (4)].
Circulating antibodies may persist for several years.
Only use Enoxaparin Sodium Injection in patients with a history of HIT, if
more than 100 days have elapsed since the prior HIT episode and no circulating
antibodies are present. Because HIT may still occur in these circumstances,
the decision to use Enoxaparin Sodium Injection
in such a case must be made only after a careful benefit-risk assessment and
after nonheparin alternative treatments are considered.
5.5 Thrombocytopenia
Thrombocytopenia can occur with the administration of Enoxaparin Sodium
Injection.
Moderate thrombocytopenia (platelet counts between 100,000/mm3 and 50,000/mm3)
occurred at a rate of 1.3% in patients given Enoxaparin Sodium Injection, 1.2%
in patients given heparin, and 0.7% in patients given placebo in clinical
trials.
Platelet counts less than 50,000/mm3 occurred at a rate of 0.1% in patients
given Enoxaparin
Sodium Injection, in 0.2% of patients given heparin, and 0.4% of patients
given placebo in the same trials.
Thrombocytopenia of any degree should be monitored closely. If the platelet count falls below 100,000/mm3, Enoxaparin Sodium Injection should be discontinued.
5.6 Interchangeability with Other Heparins
Enoxaparin Sodium Injection cannot be used interchangeably (unit for unit) with heparin or other low molecular weight heparins as they differ in manufacturing process, molecular weight distribution, anti-Xa and anti-IIa activities, units, and dosage. Each of these medicines has its own instructions for use.
5.7 Increased Risk of Thrombosis in Pregnant Women with Mechanical
Prosthetic Heart Valves
Use of Enoxaparin Sodium Injection for thromboprophylaxis in pregnant women with mechanical prosthetic heart valves may result in valve thrombosis. In a clinical study of pregnant women with mechanical prosthetic heart valves given Enoxaparin Sodium Injection (1 mg/kg twice daily) to reduce the risk of thromboembolism, 2 of 8 women developed clots resulting in blockage of the valve and leading to maternal and fetal death. No patients in the heparin/warfin group (0 of 4 women) died. There also have been isolated postmarketing reports of valve thrombosis in pregnant women with mechanical prosthetic heart valves while receiving Enoxaparin Sodium Injection for thromboprophylaxis. Women with mechanical prosthetic heart valves may be at higher risk for thromboembolism during pregnancy, and, when pregnant, have a higher rate of fetal loss from stillbirth, spontaneous abortion and premature delivery. Therefore, frequent monitoring of peak and trough anti-Factor Xa levels, and adjusting of dosage may be needed [see Use in Specific Populations (8.6)].
5.8 Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol
Preservative
Enoxaparin Sodium Injection multiple-dose vials are not approved for use in
neonates or infants. Serious and fatal adverse reactions including “gasping
syndrome” can occur in neonates and low birth weight infants treated with
benzyl alcohol-preserved drugs, including Enoxaparin Sodium Injection
multiple-dose vials. The “gasping syndrome” is characterized by central
nervous system depression, metabolic acidosis, and gasping respirations. The
minimum amount of benzyl alcohol at which serious adverse reactions may occur
is not known
(Enoxaparin Sodium Injection multiple-dose vials contain 15 mg of benzyl
alcohol per mL) [see Use in Specific Populations (8.4)].
Because benzyl alcohol may cross the placenta, if anticoagulation with
Enoxaparin Sodium Injection is needed during pregnancy, use the preservative-
free formulations where possible [see Use in Specific Populations (8.1)].
- Increased Risk of Hemorrhage: Monitor for signs of bleeding. (5.1, 5.2, 5.3)
- Risk of Heparin-Induced Thrombocytopenia with or without Thrombosis. (5.4)
- Thrombocytopenia: Monitor platelet count closely. (5.5)
- Interchangeability with other heparins: Do not exchange with heparin or other LMWHs. (5.6)
- Increased Risk of Thrombosis in Pregnant Women with Mechanical Prosthetic Heart Valves: Women and their fetuses may be at increased risk. Monitor more frequently and adjust dosage as needed. (5.7)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
Whenever possible, agents which may enhance the risk of hemorrhage should be discontinued prior to initiation of Enoxaparin Sodium Injection therapy. These agents include medications such as: anticoagulants, platelet inhibitors including acetylsalicylic acid, salicylates, NSAIDs (including ketorolac tromethamine), dipyridamole, or sulfinpyrazone. If coadministration is essential, conduct close clinical and laboratory monitoring [see Warnings and Precautions (5.1)].
Discontinue agents which may enhance hemorrhage risk prior to initiation of Enoxaparin Sodium Injection or conduct close clinical and laboratory monitoring. (2.6, 7)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Enoxaparin Sodium Injection USP is a clear, colorless to pale yellow solution available in two concentrations.
100 mg/mL Concentration
|
-Single-Dose Prefilled Syringes |
30 mg / 0.3 mL, 40 mg / 0.4 mL |
|
-Single-Dose Graduated Prefilled Syringes |
60 mg / 0.6 mL, 80 mg / 0.8 mL, 100 mg / 1 mL |
|
-Multiple-Dose Vials |
300 mg / 3 mL |
150 mg/mL Concentration
|
-Single-Dose Graduated Prefilled Syringes |
120 mg / 0.8 mL, 150 mg / 1 mL |
100 mg/mL concentration (3):
• Single-dose prefilled syringes: 30 mg/0.3 mL, 40 mg/0.4 mL
• Single-dose graduated prefilled syringes: 60 mg/0.6 mL, 80 mg/0.8 mL, 100
mg/1 mL
• Multiple-dose vial: 300 mg/3 mL
150 mg/mL concentration (3):
• Single-dose graduated prefilled syringes: 120 mg/0.8 mL, 150 mg/1 mL
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Placental transfer of enoxaparin was observed in the animal studies. Human
data from a retrospective cohort study, which included 693 live births,
suggest that enoxaparin does not increase the risk of major developmental
abnormalities (see Data). Based on animal data, Enoxaparin Sodium Injection is
not predicted to increase the risk of major developmental abnormalities (see
Data).
Adverse outcomes in pregnancy occur regardless of the health of the mother or
the use of medications. The estimated background risk of major birth defects
and miscarriage for the indicated populations is unknown. In the U.S. general
population, the estimated background
risk of major birth defects and miscarriage in clinically recognized
pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Pregnancy alone confers an increased risk for thromboembolism that is even
higher for women with thromboembolic disease and certain high risk pregnancy
conditions. While not adequately studied, pregnant women with mechanical
prosthetic heart valves may be at even higher risk for thrombosis [see Warnings and Precautions (5.7) and Use in Specific Populations (8.6)].
Pregnant women with thromboembolic disease, including those with mechanical
prosthetic heart valves and those with inherited or acquired thrombophilias,
have an increased risk of other maternal complications and fetal loss
regardless of the type of anticoagulant used.
All patients receiving anticoagulants, including pregnant women, are at risk
for bleeding. Pregnant women receiving Enoxaparin Sodium Injection should be
carefully monitored for evidence of bleeding or excessive anticoagulation.
Consideration for use of a shorter acting anticoagulant should be specifically
addressed as delivery approaches [see Boxed Warning]. Hemorrhage can occur at
any site and may lead to death of mother and/or fetus. Pregnant women should
be apprised of the potential hazard to the fetus and the mother if Enoxaparin
Sodium Injection is administered during pregnancy.
It is not known if monitoring of anti-Factor Xa activity and dose adjustment
(by weight or anti-Factor Xa activity) of Enoxaparin Sodium Injection affect
the safety and the efficacy of the drug during pregnancy.
Cases of “gasping syndrome” have occurred in premature infants when large
amounts of benzyl alcohol have been administered (99-405 mg/kg/day). The
multiple-dose vial of Enoxaparin Sodium Injection contains 15 mg benzyl
alcohol per 1 mL as a preservative [see Warnings and Precautions (5.8)].
Data
Human Data
There are no adequate and well-controlled studies in pregnant women. A
retrospective study reviewed the records of 604 women who used Enoxaparin
Sodium Injection during pregnancy. A total of 624 pregnancies resulted in 693
live births. There were 72 hemorrhagic
events (11 serious) in 63 women. There were 14 cases of neonatal hemorrhage.
Major congenital anomalies in live births occurred at rates (2.5%) similar to
background rates. There have been postmarketing reports of fetal death when
pregnant women received Enoxaparin Sodium Injection. Causality for these cases
has not been determined.
Insufficient data, the underlying disease, and the possibility of inadequate
anticoagulation complicate the evaluation of these cases.
A clinical study using Enoxaparin Sodium Injection in pregnant women with
mechanical prosthetic heart valves has been conducted [see Warnings and Precautions (5.7)].
Animal data
Teratology studies have been conducted in pregnant rats and rabbits at
subcutaneous doses of enoxaparin up to 15 times the recommended human dose (by
comparison with 2 mg/kg as the maximum recommended daily dose). There was no
evidence of teratogenic effects or fetotoxicity due to enoxaparin. Because
animal reproduction studies are not always predictive of human response, this
drug should be used during pregnancy only if clearly needed.
8.2 Lactation
Risk Summary
It is unknown whether Enoxaparin Sodium Injection is excreted in human milk.
In lactating rats, the passage of enoxaparin or its metabolites in the milk is
very limited. There is no information available on the effect of enoxaparin or
its metabolites on the breastfed child, or on the milk production. The
developmental and health benefits of breastfeeding should be considered along
with the mother’s clinical need for Enoxaparin Sodium Injection and any
potential adverse effects on the breastfed child from Enoxaparin Sodium
Injection or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of Enoxaparin Sodium Injection in pediatric patients
have not been
established.
Enoxaparin Sodium Injection is not approved for use in neonates or infants.
Serious adverse reactions including fatal reactions and the “gasping syndrome”
occurred in premature neonates and low birth weight infants in the neonatal
intensive care unit who received drugs containing benzyl alcohol as a
preservative. In these cases, benzyl alcohol dosages of 99 to 234 mg/kg/day
produced high levels of benzyl alcohol and its metabolites in the blood and
urine (blood levels of benzyl alcohol were 0.61 to 1.378 mmol/L). Additional
adverse reactions included gradual neurological deterioration, seizures,
intracranial
hemorrhage, hematologic abnormalities, skin breakdown, hepatic and renal
failure, hypotension, bradycardia, and cardiovascular collapse. Preterm, low-
birth weight infants may be more likely to develop these reactions because
they may be less able to metabolize benzyl alcohol. The minimum amount of
benzyl alcohol at which serious adverse reactions may occur is not known.
Enoxaparin Sodium Injection multiple-dose vials contain 15 mg/mL of benzyl
alcohol (at the dose of 1.5 mg/kg twice a day, benzyl alcohol exposure in
patients is 0.45 mg/kg daily) [see Warnings and Precautions (5.8)].
8.5 Geriatric Use
Prevention of Deep Vein Thrombosis in Hip, Knee and Abdominal Surgery;
Treatment of Deep Vein Thrombosis, Prevention of Ischemic Complications of
Unstable Angina and Non-Q-Wave Myocardial Infarction
Over 2800 patients, 65 years and older, have received Enoxaparin Sodium
Injection in clinical trials. The efficacy of Enoxaparin Sodium Injection in
the geriatric (≥65 years) was similar to that seen in younger patients (<65
years). The incidence of bleeding complications was similar between geriatric
and younger patients when 30 mg every 12 hours or 40 mg once a day doses of
Enoxaparin Sodium Injection were employed. The incidence of bleeding
complications was higher in geriatric patients as compared to younger patients
when Enoxaparin Sodium Injection was administered at doses of 1.5 mg/kg once a
day or 1 mg/kg every 12 hours. The risk of Enoxaparin Sodium Injection-
associated bleeding increased with age. Serious adverse events increased with
age for patients receiving Enoxaparin Sodium Injection. Other clinical
experience (including postmarketing surveillance and literature reports) has
not revealed additional differences in the safety of Enoxaparin Sodium
Injection between geriatric and younger patients. Careful attention to dosing
intervals and concomitant medications (especially antiplatelet medications) is
advised. Enoxaparin Sodium Injection should be used with care in geriatric
patients who may show delayed elimination of enoxaparin. Monitoring of
geriatric patients with low body weight (<45 kg) and those predisposed to
decreased renal function should be considered [see Warnings and Precautions (2.6) and Clinical Pharmacology (12.3)].
Treatment of Acute ST-Segment Elevation Myocardial Infarction
In the clinical study for treatment of acute ST-segment elevation myocardial
infarction, there was no evidence of difference in efficacy between patients
≥75 years of age (n = 1241) and patients less than 75 years of age (n = 9015).
Patients ≥75 years of age did not receive a 30 mg intravenous bolus prior to
the normal dosage regimen and had their subcutaneous dose adjusted to 0.75
mg/kg every 12 hours [see Dosage and Administration (2.4)]. The incidence of
bleeding complications was higher in patients ≥65 years of age as compared to
younger patients (<65 years).
8.6 Patients with Mechanical Prosthetic Heart Valves
The use of Enoxaparin Sodium Injection has not been adequately studied for thromboprophylaxis in patients with mechanical prosthetic heart valves and has not been adequately studied for long-term use in this patient population. Isolated cases of prosthetic heart valve thrombosis have been reported in patients with mechanical prosthetic heart valves who have received Enoxaparin Sodium Injection for thromboprophylaxis. Some of these cases were pregnant women in whom thrombosis led to maternal and fetal deaths. Insufficient data, the underlying disease and the possibility of inadequate anticoagulation complicate the evaluation of these cases. Pregnant women with mechanical prosthetic heart valves may be at higher risk for thromboembolism [see Warnings and Precautions (5.7)].
8.7 Renal Impairment
In patients with renal impairment, there is an increase in exposure of enoxaparin sodium. All such patients should be observed carefully for signs and symptoms of bleeding. Because exposure of enoxaparin sodium is significantly increased in patients with severe renal impairment (creatinine clearance <30 mL/min), a dosage adjustment is recommended for therapeutic and prophylactic dosage ranges. No dosage adjustment is recommended in patients with creatinine clearance 30 to <50 mL/min and creatinine clearance 50 to 80 mL/min [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)]. In patients with renal failure, treatment with Enoxaparin Sodium Injection has been associated with the development of hyperkalemia [see Adverse Reactions (6.2)].
8.8 Low-Weight Patients
An increase in exposure of enoxaparin sodium with prophylactic dosages (non- weight adjusted) has been observed in low-weight women (<45 kg) and low-weight men (<57 kg). Observe low-weight patients frequently for signs and symptoms of bleeding [see Clinical Pharmacology (12.3)].
8.9 Obese Patients
Obese patients are at higher risk for thromboembolism. The safety and efficacy of prophylactic doses of Enoxaparin Sodium Injection in obese patients (BMI
30 kg/m2) has not been fully determined and there is no consensus for dose adjustment. Observe these patients carefully for signs and symptoms of thromboembolism.
• Severe Renal Impairment: Adjust dose for patients with creatinine clearance
<30 mL/min. (2.3, 8.7)
• Geriatric Patients: Monitor for increased risk of bleeding. (8.5)
• Low-Weight Patients: Observe for signs of bleeding. (8.8)
DESCRIPTION SECTION
11 DESCRIPTION
Enoxaparin Sodium Injection USP is a sterile aqueous solution containing
enoxaparin sodium, a low molecular weight heparin. The pH of the injection is
5.5 to 7.5.
Enoxaparin sodium is obtained by alkaline depolymerization of heparin benzyl
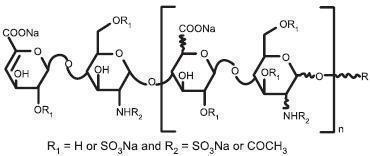
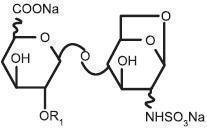
ester derived from porcine intestinal mucosa. Its structure is characterized
by a 2-O-sulfo-4-enepyranosuronic acid group at the non-reducing end and a
2-N,6-O-disulfo-D-glucosamine at the reducing end of the chain. About 20%
(ranging between 15% and 25%) of the enoxaparin structure contains a 1,6
anhydro derivative on the reducing end of the polysaccharide chain. The drug
substance is the sodium salt. The average molecular weight is about 4500
daltons. The molecular weight distribution is:
|
<2000 daltons |
20% |
|
2000 to 8000 daltons |
68% |
|
18% |
| |||
|
STRUCTURAL FORMULA
| |||
|
R |
X*******=15 to 25%** |
|
n=0 to 20 |
|
100 - X |
H |
n=1 to 21 |
Enoxaparin Sodium Injection USP 100 mg/mL Concentration contains 10 mg enoxaparin sodium (approximate anti-Factor Xa activity of 1000 IU [with reference to the W.H.O. First International Low Molecular Weight Heparin Reference Standard]) per 0.1 mL Water for Injection.
Enoxaparin Sodium Injection USP 150 mg/mL Concentration contains 15 mg
enoxaparin sodium (approximate anti-Factor Xa activity of 1500 IU [with reference to the W.H.O. First International Low Molecular Weight Heparin Reference Standard]) per 0.1 mL Water for Injection.
The Enoxaparin Sodium Injection USP prefilled syringes and graduated prefilled
syringes are preservative-free and intended for use only as a single-dose
injection. The multiple-dose vial contains 15 mg benzyl alcohol per 1 mL as a
preservative [see Dosage and Administration (2) and How Supplied/Storage and Handling(16)].
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Enoxaparin is a low molecular weight heparin, which has antithrombotic properties.
12.2 Pharmacodynamics
In humans, enoxaparin given at a dose of 1.5 mg/kg subcutaneously is characterized by a higher ratio of anti-Factor Xa to anti-Factor IIa activity (mean ±SD, 14.0±3.1) (based on areas under anti-Factor activity versus time curves) compared to the ratios observed for heparin (mean ±SD, 1.22±0.13). Increases of up to 1.8 times the control values were seen in the thrombin time (TT) and the activated partial thromboplastin time (aPTT). Enoxaparin at a 1 mg/kg dose (100 mg/mL concentration), administered subcutaneously every 12 hours to patients in a large clinical trial resulted in aPTT values of 45 seconds or less in the majority of patients (n=1607). A 30 mg intravenous bolus immediately followed by a 1 mg/kg subcutaneous administration resulted in aPTT postinjection values of 50 seconds. The average aPTT prolongation value on Day 1 was about 16% higher than on Day 4.
12.3 Pharmacokinetics
Absorption
Pharmacokinetic trials were conducted using the 100 mg/mL formulation. Maximum
anti-Factor Xa and anti-thrombin (anti-Factor IIa) activities occur 3 to 5
hours after subcutaneous injection of enoxaparin. Mean peak anti-Factor Xa
activity was 0.16 IU/mL (1.58 mcg/mL) and 0.38 IU/mL (3.83 mcg/mL) after the
20 mg and the 40 mg clinically tested subcutaneous doses, respectively. Mean
(n = 46) peak anti-Factor Xa activity was 1.1 IU/mL at steady state in
patients with unstable angina receiving 1 mg/kg subcutaneously every 12 hours
for 14 days. Mean absolute bioavailability of enoxaparin, after 1.5 mg/kg
given subcutaneously, based on anti-Factor Xa activity is approximately 100%
in healthy subjects.
A 30 mg intravenous bolus immediately followed by a 1 mg/kg subcutaneously
every 12 hours provided initial peak anti-Factor Xa levels of 1.16 IU/mL
(n=16) and average exposure corresponding to 84% of steady-state levels.
Steady state is achieved on the second day of treatment.
Enoxaparin pharmacokinetics appear to be linear over the recommended dosage
ranges [see Dosage and Administration (2)]. After repeated subcutaneous
administration of 40 mg once daily and 1.5 mg/kg once-daily regimens in
healthy volunteers, the steady state is reached on day 2 with an average
exposure ratio about 15% higher than after a single dose. Steady-state
enoxaparin activity levels are well predicted by single-dose pharmacokinetics.
After repeated subcutaneous administration of the 1 mg/kg twice-daily regimen,
the steady state is reached from day 4 with mean exposure about 65% higher
than after a single dose and mean peak and trough levels of about 1.2 and 0.52
IU/mL, respectively. Based on enoxaparin sodium pharmacokinetics, this
difference in steady state is expected and within the therapeutic range.
Although not studied clinically, the 150 mg/mL concentration of enoxaparin
sodium is projected to result in anticoagulant activities similar to those of
100 mg/mL and 200 mg/mL concentrations at the same enoxaparin dose. When a
daily 1.5 mg/kg subcutaneous injection of enoxaparin sodium was given to 25
healthy male and female subjects using a 100 mg/mL or a 200 mg/mL
concentration the following pharmacokinetic profiles were obtained (see Table
13).
|
Concentration |
Anti-Xa |
Anti-IIa |
Heptest |
aPTT | |
|---|---|---|---|---|---|
| |||||
|
Amax |
100 mg/mL |
1.37 (±0.23) |
0.23 (±0.05) |
105 (±17) |
19 (±5) |
|
200 mg/mL |
1.45 (±0.22) |
0.26 (±0.05) |
111 (±17) |
22 (±7) | |
|
90% CI |
102%-110% |
102%-111% | |||
|
tmax*(h) |
100 mg/mL |
3 (2-6) |
4 (2-5) |
2.5 (2-4.5) |
3 (2-4.5) |
|
200 mg/mL |
3.5 (2-6) |
4.5 (2.5-6) |
3.3 (2-5) |
3 (2-5) | |
|
AUC (ss) |
100 mg/mL |
14.26 (±2.93) |
1.54 (±0.61) |
1321 (±219) | |
|
200 mg/mL |
15.43 (±2.96) |
1.77 (±0.67) |
1401 (±227) | ||
|
90% CI |
105%-112% |
103%-109% |
Distribution
The volume of distribution of anti-Factor Xa activity is about 4.3 L.
Elimination
Following intravenous dosing, the total body clearance of enoxaparin is 26
mL/min. After intravenous dosing of enoxaparin labeled with the gamma-emitter,
99mTc, 40% of radioactivity and 8 to 20% of anti-Factor Xa activity were
recovered in urine in 24 hours. Elimination half-life based on anti-Factor Xa
activity was 4.5 hours after a single subcutaneous dose to about 7 hours after
repeated dosing. Significant anti-Factor Xa activity persists in plasma for
about 12 hours following a 40 mg subcutaneous once a day dose.
Following subcutaneous dosing, the apparent clearance (CL/F) of enoxaparin is
approximately 15 mL/min.
Metabolism
Enoxaparin sodium is primarily metabolized in the liver by desulfation and/or
depolymerization to lower molecular weight species with much reduced
biological potency. Renal clearance of active fragments represents about 10%
of the administered dose and total renal excretion of active and non-active
fragments 40% of the dose.
Special Populations
Gender
Apparent clearance and Amax derived from anti-Factor Xa values following
single subcutaneous dosing (40 mg and 60 mg) were slightly higher in males
than in females. The source of the gender difference in these parameters has
not been conclusively identified; however, body weight may be a contributing
factor.
Geriatric
Apparent clearance and Amax derived from anti-Factor Xa values following
single and multiple subcutaneous dosing in geriatric subjects were close to
those observed in young subjects. Following once a day subcutaneous dosing of
40 mg enoxaparin, the Day 10 mean area under anti-Factor Xa activity versus
time curve (AUC) was approximately 15% greater than the mean Day 1 AUC value
[see Dosage and Administration (2.4) and Use in Specific Populations (8.5)].
Renal impairment
A linear relationship between anti-Factor Xa plasma clearance and creatinine
clearance at steady state has been observed, which indicates decreased
clearance of enoxaparin sodium in patients with reduced renal function. Anti-
Factor Xa exposure represented by AUC, at
steady state, is marginally increased in patients with creatinine clearance 50
to 80 mL/min and patients with creatinine clearance 30 to <50 mL/min after
repeated subcutaneous 40 mg once-daily doses. In patients with severe renal
impairment (creatinine clearance <30 mL/min), the AUC at steady state is
significantly increased on average by 65% after repeated subcutaneous 40 mg
once-daily doses [see Dosage and Administration (2.3) and Use in Specific Populations (8.7)].
Hemodialysis
In a single study, elimination rate appeared similar but AUC was two-fold
higher than control population, after a single 0.25 or 0.5 mg/kg intravenous
dose.
Hepatic Impairment
Studies with Enoxaparin Sodium Injection in patients with hepatic impairment
have not been conducted and the impact of hepatic impairment on the exposure
to enoxaparin is unknown.
Weight
After repeated subcutaneous 1.5 mg/kg once daily dosing, mean AUC of anti-
Factor Xa activity is marginally higher at steady state in obese healthy
volunteers (BMI 30-48 kg/m2) compared to non-obese control subjects, while
Amax is not increased.
When non-weight adjusted dosing was administered, it was found after a single-
subcutaneous 40 mg dose, that anti-Factor Xa exposure is 52% higher in low-
weight women (<45 kg) and 27% higher in low-weight men (< 57 kg) when compared
to normal weight control subjects [see Use in Specific Populations (8.8)].
Pharmacokinetic interaction
No pharmacokinetic interaction was observed between Enoxaparin Sodium
Injection and thrombolytics when administered concomitantly.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies in animals have been performed to evaluate the carcinogenic potential of enoxaparin. Enoxaparin was not mutagenic in in vitro tests, including the Ames test, mouse lymphoma cell forward mutation test, and human lymphocyte chromosomal aberration test, and the in vivo rat bone marrow chromosomal aberration test. Enoxaparin was found to have no effect on fertility or reproductive performance of male and female rats at subcutaneous doses up to 20 mg/kg/day or 141 mg/m2/day. The maximum human dose in clinical trials was 2.0 mg/kg/day or 78 mg/m2/day (for an average body weight of 70 kg, height of 170 cm, and body surface area of 1.8 m2).
13.2 Animal Toxicology and/or Pharmacology
A single subcutaneous dose of 46.4 mg/kg enoxaparin was lethal to rats. The symptoms of acute toxicity were ataxia, decreased motility, dyspnea, cyanosis, and coma.
13.3 Reproductive and Developmental Toxicology
Teratology studies have been conducted in pregnant rats and rabbits at subcutaneous doses of enoxaparin up to 30 mg/kg/day corresponding to 211 mg/m2/day and 410 mg/m2/day in rats and rabbits respectively. There was no evidence of teratogenic effects or fetotoxicity due to enoxaparin.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Enoxaparin Sodium Injection USP is available in two concentrations (see Tables 26 and 27)
|
Table 26 100 mg/mL Concentration | ||||
|---|---|---|---|---|
|
Dosage Unit/Strength* |
Anti-Xa Activity |
Package Size |
Syringe Label Color |
NDC# 0548- |
| ||||
|
Single-Dose Prefilled Syringes**†** |
3000 IU |
10 syringes |
Medium |
5601-00 |
|
40 mg/0.4 mL |
4000 IU |
10 syringes |
Yellow |
5602-00 |
|
Single-Dose Graduated Prefilled Syringes**‡** | ||||
|
60 mg/0.6 mL |
6000 IU |
10 syringes |
Orange |
5603-00 |
|
80 mg/0.8 mL |
8000 IU |
10 syringes |
Brown |
5604-00 |
|
100 mg/1 mL |
10,000 IU |
10 syringes |
Black |
5605-00 |
|
Multiple-Dose Vial**§** | ||||
|
300 mg/3 mL |
30,000 IU |
1 vial |
Red |
5608-00 |
|
Table 27 150 mg/mL Concentration | ||||
|---|---|---|---|---|
|
Dosage Unit /Strength* |
Anti-Xa Activity† |
Package Size |
Syringe Label Color |
NDC # 0548- |
| ||||
|
Single-Dose Graduated Prefilled Syringes**‡** | ||||
|
120 mg/0.8 mL |
12,000 IU |
10 syringes |
Purple |
5606-00 |
|
150 mg/1 mL |
15,000 IU |
10 syringes |
Navy Blue |
5607-00 |
Store at 25°C (77°F); excursions permitted to 15°C - 30°C (59°F - 86°F) [See USP Controlled Room Temperature]. Store in the original carton or packaging
until ready to use.
Do not store the multiple-dose vials for more than 28 days after the first
use.