Numbness Cream
84938-002 Numbness Cream
3605961a-d596-9c4a-e063-6394a90a99dc
HUMAN OTC DRUG LABEL
May 26, 2025
Foshan Sugar Max Cosmetics CO.,Ltd
DUNS: 700689935
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Lidocaine
PRODUCT DETAILS
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
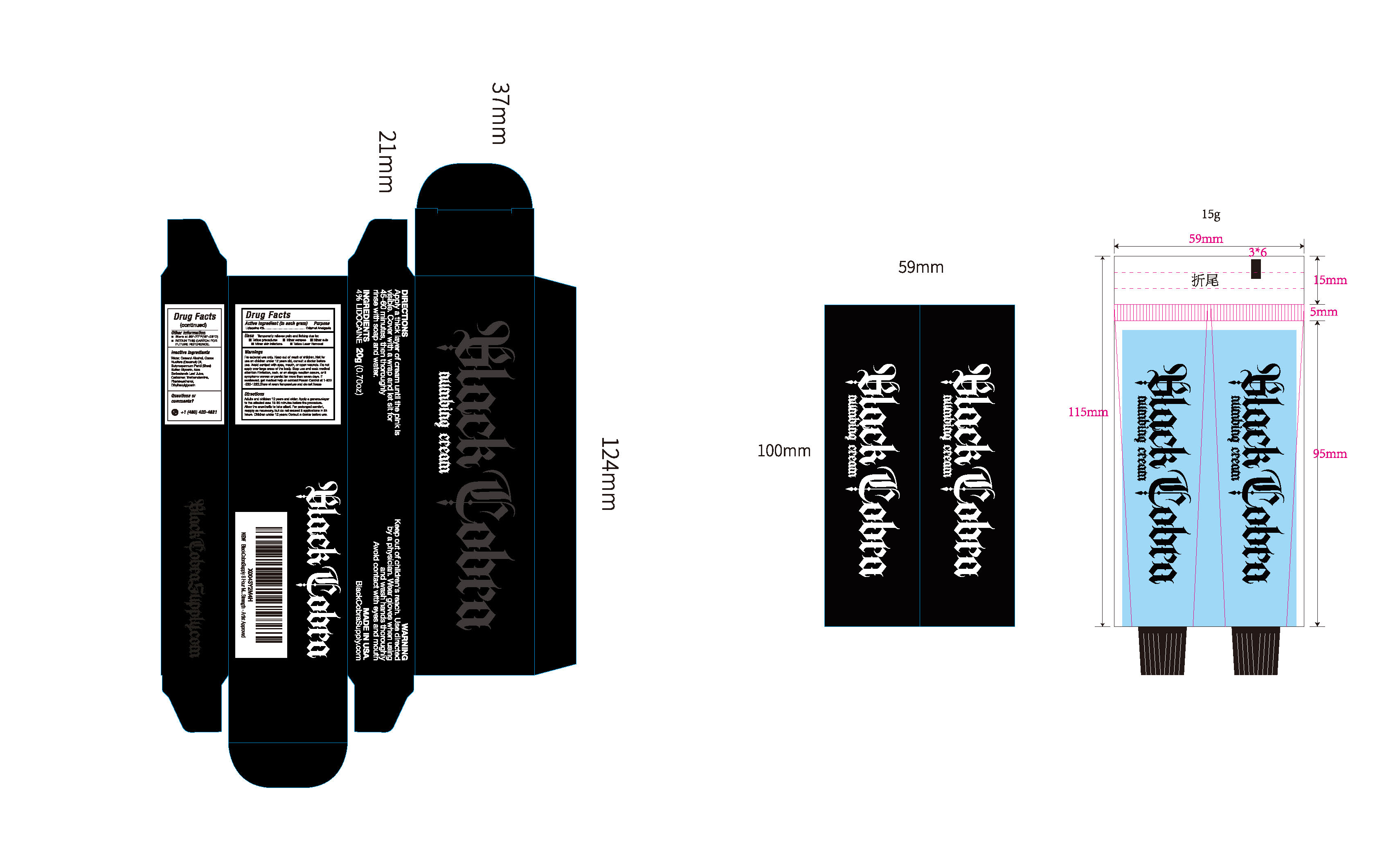
INDICATIONS & USAGE SECTION
Temporarily relieves pain and itching due to: Tattoo procedures
Minor scrapes, Minor cuts, Minor skin irritations,
Tattoo Laser Removal
OTC - STOP USE SECTION
Stop use and seek medicaattention if irritation, rash, or an allergic reaction occurs, or if symptoms worsen or persist for more than seven days.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
lf swallowed, get medical help or contact Poison Control at 1-800-222-1222.Store at room temperature and do not freeze
DOSAGE & ADMINISTRATION SECTION
Adults and children 12 years and older: Apply a generouslayerto the affected area 15-30 minutes before the procedure.Allow the anesthetic to take effect. For prolonged comfort,reapply as necessary, but do not exceed 3 applications in 24hours. Children under 12 years: Consult a doctor before use.
INACTIVE INGREDIENT SECTION
Aqua
Cetearyl Alcohol
Cocos Nucifera (Coconut) Oil
Butyrospermum Parkii (Shea) Butter
Glycerin
Aloe Barbadensis Leaf Juice
Carbomer
Triethanolamine
Phenoxyethanol
Ethylhexylglycerin
OTC - ACTIVE INGREDIENT SECTION
Lidocaine 4%
OTC - PURPOSE SECTION
External Analgesic
WARNINGS SECTION
For external use only.
OTC - WHEN USING SECTION
Not foruse on children under 12 years old, consult a doctor beforeuse.
Avoid contact with eyes, mouth, or open wounds.
