Glycerin
Glycerin Suppositories 2 g
db539b39-9504-44d2-9bd5-ba6ffb441024
HUMAN OTC DRUG LABEL
Sep 8, 2025
Rising Pharma Holdings, Inc.
DUNS: 116880195
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Glycerin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 57237-325-52 (5 blisters of 5 suppositories)
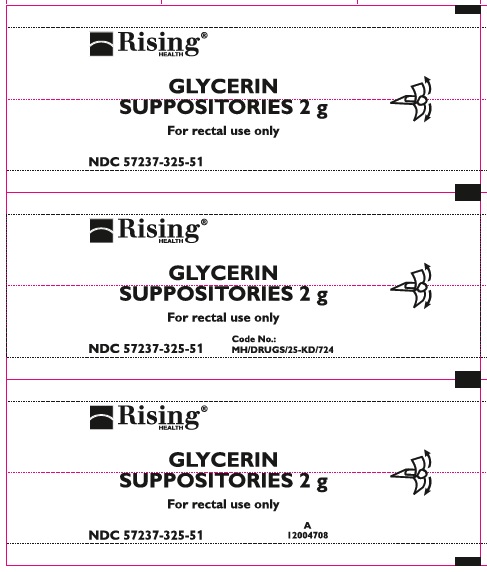
GLYCERIN SUPPOSITORIES 2 g
LAXATIVE
For fast, gentle relief of occasional constipation


NDC 57237-325-51 (5 suppositories in 1 blister pack)
Front Foil
Back Foil
INDICATIONS & USAGE SECTION
**Uses**
- for relief of occasional constipation
- this product usually produces bowel movement in 1/4 to 1 hour
SPL UNCLASSIFIED SECTION
Distributed by:
Rising Pharma Holdings, Inc.
East Brunswick, NJ 08816
Made in India
CODE NO.: MH/DRUGS/25-KD/724
Issued: 08/2023
OTC - ACTIVE INGREDIENT SECTION
Active ingredient (in each suppository)
Glycerin USP, 2 grams
OTC - PURPOSE SECTION
Purpose
Laxative
WARNINGS SECTION
Warnings
**For rectal use only**
Do not use for longer than 1 week unless directed by a doctor
Ask a doctor before use if you have
- abdominal pain, nausea or vomiting
- a sudden change in bowel habits lasting more than 2 weeks
When using this productit may cause rectal discomfort or a burning sensation
Stop use and ask a doctor if you have
- rectal bleeding
- no bowel movement after using this product
These may indicate a serious condition.
**Keep out of reach of children.**
If swallowed, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
- adults and children 6 years of age and over : 1 suppository once daily
- children under 6 years : do not use
- insert 1 suppository well up into rectum and retain for 15 minutes
- it need not melt to produce laxative action
STORAGE AND HANDLING SECTION
Other information
keep away from excessive heat.
INACTIVE INGREDIENT SECTION
Inactive ingredients
purified water, sodium stearate
OTC - QUESTIONS SECTION
Questions or Comments?
Call1-844-474-7464
