Irbesartan and Hydrochlorothiazide
These highlights do not include all the information needed to use IRBESARTAN and HYDROCHLOROTHIAZIDE TABLETS safely and effectively. See full prescribing information for IRBESARTAN and HYDROCHLOROTHIAZIDE TABLETS. IRBESARTAN and HYDROCHLOROTHIAZIDE Tablets, for oral use Initial U.S. Approval: 1997
e842fa14-b18e-4463-9bf1-a1c8700fe33c
HUMAN PRESCRIPTION DRUG LABEL
Sep 5, 2023
Alembic Pharmaceuticals Inc.
DUNS: 079288842
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Irbesartan and Hydrochlorothiazide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Irbesartan and Hydrochlorothiazide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Irbesartan and Hydrochlorothiazide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL 300 mg 25 mg
NDC 62332-053-30****
Irbesartan and
Hydrochlorothiazide
Tablets, USP
300 mg/25 mg
Rx only
****30 Tablets
Alembic

Boxed Warning Section
** WARNING: FETAL TOXICITY**
****See full prescribing information for complete boxed warning.
Indications & Usage Section
1. INDICATIONS AND USAGE
Irbesartan and Hydrochlorothiazide Tablets are indicated for the treatment of
hypertension.
Irbesartan and Hydrochlorothiazide Tablets may be used in patients whose blood
pressure is not adequately controlled on monotherapy.
Irbesartan and Hydrochlorothiazide Tablets may also be used as initial therapy
in patients who are likely to need multiple drugs to achieve their blood
pressure goals.
The choice of Irbesartan and Hydrochlorothiazide Tablets as initial therapy
for hypertension should be based on an assessment of potential benefits and
risks.
Patients with stage 2 (moderate or severe) hypertension are at relatively high
risk for cardiovascular events (such as strokes, heart attacks, and heart
failure), kidney failure, and vision problems, so prompt treatment is
clinically relevant. The decision to use a combination as initial therapy
should be individualized and may be shaped by considerations such as the
baseline blood pressure, the target goal, and the incremental likelihood of
achieving goal with a combination compared with mono therapy.
Data from Studies V and VI [see Clinical Studies (14.2)] provide estimates of
the probability of reaching a blood pressure goal with Irbesartan and
Hydrochlorothiazide Tablets compared to irbesartan or hydrochlorothiazide
(HCTZ) monotherapy. The relationship between baseline blood pressure and
achievement of a SeSBP <140 or <130 mmHg or SeDBP <90 or <80 mmHg in patients
treated with Irbesartan and Hydrochlorothiazide Tablets compared to patients
treated with irbesartan or HCTZ monotherapy are shown in Figures 1a through
2b.
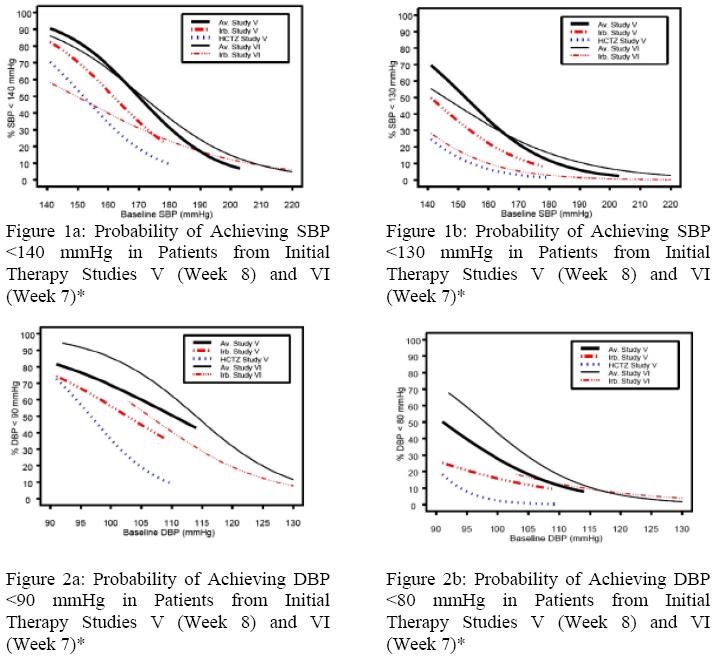
*For all probability curves, patients without blood pressure measurements at Week 7 (Study VI) and Week 8 (Study V) were counted as not reaching goal (intent-to- treat analysis).
The above graphs provide a rough approximation of the likelihood of reaching a
targeted blood pressure goal (e.g., Week 8 sitting systolic blood pressure
≤140 mmHg) for the treatment groups. The curve of each treatment group in each
study was estimated by logistic regression modeling from all available data of
that treatment group. The estimated likelihood at the right tail of each curve
is less reliable due to small numbers of subjects with high baseline blood
pressures.
For example, a patient with a blood pressure of 180/105 mmHg has about a 25%
likelihood of achieving a goal of <140 mmHg (systolic) and 50% likelihood of
achieving <90 mmHg (diastolic) on irbesartan alone (and lower still
likelihoods on HCTZ alone).
The likelihood of achieving these goals on Irbesartan and Hydrochlorothiazide
Tablets rises to about 40% (systolic) or 70% (diastolic).
Irbesartan and Hydrochlorothiazide Tablets USP is a combination of irbesartan, an angiotensin II receptor antagonist, and hydrochlorothiazide, a thiazide diuretic, indicated for hypertension: (1)
- In patients not adequately controlled with monotherapy. (1)
- As initial therapy in patients likely to need multiple drugs to achieve their blood pressure goals. (1)
Information For Patients Section
17. PATIENT COUNSELING INFORMATION
Pregnancy
Tell female patients of childbearing age about the consequences of exposure to Irbesartan and Hydrochlorothiazide Tablets during pregnancy. Discuss treatment options with women planning to become pregnant. Ask patients to report pregnancies to their physician as soon as possible.
Symptomatic Hypotension
Tell patients using Irbesartan and Hydrochlorothiazide Tablets that they may feel lightheaded, especially during the first days of use. Tell patients to inform their physician if they feel lightheaded or faint. Tell the patient, if fainting occurs, stop using Irbesartan and Hydrochlorothiazide Tablets and contact the prescribing doctor.
Tell patients using Irbesartan and Hydrochlorothiazide Tablets that getting dehydrated can lower their blood pressure too much and lead to lightheadedness and possible fainting. Dehydration may occur with excessive sweating, diarrhea, or vomiting and with not drinking enough liquids.
Potassium Supplements
Advise patients not to use potassium supplements or salt substitutes containing potassium without consulting their healthcare provider [see Drug Interactions (7.3)].
Acute Angle-Closure Glaucoma, Acute Myopia, and Choroidal Effusion
Advise patients to discontinue Irbesartan and Hydrochlorothiazide Tablets and seek immediate medical attention if they experience symptoms of acute angle- closure glaucoma, acute myopia, and choroidal effusion [see Warnings and Precautions (5.8)].
Non-melanoma Skin Cancer
Instruct patients taking hydrochlorothiazide to protect skin from the sun and undergo regular skin cancer screening.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
For more information, you can also call Alembic Pharmaceuticals Limited at 1-866-210-9797.
Manufactured by:
Alembic Pharmaceuticals Limited
(Formulation Division),
Panelav 389350, Gujarat, India
Manufactured for:
Alembic Pharmaceuticals, Inc.
Bedminster, NJ 07921, USA
Revised: 09/2023
Recent Major Changes Section
Description Section
11. DESCRIPTION
Irbesartan and Hydrochlorothiazide Tablets are a combination of an angiotensin
II receptor antagonist (AT1 subtype), irbesartan, and a thiazide diuretic,
hydrochlorothiazide (HCTZ). Irbesartan is a non-peptide compound, chemically
described as a 2-butyl-3-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]-1,3-diazaspiro
[4.4]non-1-en-4-one. Its empirical formula is C25H28N6O, and its structural
formula is:
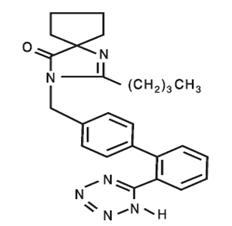
Irbesartan is a white to off-white crystalline powder with a molecular weight of 428.5. It is a nonpolar compound with a partition coefficient (octanol/water) of 10.1 at pH of 7.4. Irbesartan is slightly soluble in alcohol and methylene chloride and practically insoluble in water.
Hydrochlorothiazide is
6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1- dioxide. Its
empirical formula is C7H8ClN3O4S2 and its structural formula is:
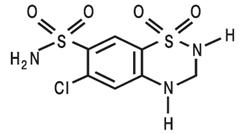
Hydrochlorothiazide is a white, or practically white, crystalline powder with
a molecular weight of 297.7
Hydrochlorothiazide is slightly soluble in water and freely soluble in sodium
hydroxide solution.
Irbesartan and Hydrochlorothiazide Tablets USP are available for oral administration in tablets containing 150 mg or 300 mg of irbesartan combined with 12.5 mg of hydrochlorothiazide or 300 mg of irbesartan combined with 25 mg hydrochlorothiazide. Inactive ingredients include: lactose monohydrate, croscarmellose sodium, povidone, magnesium stearate, iron oxide red and iron oxide yellow. In addition, the 300/25 mg pinkish brown film-coated tablet contains titanium dioxide, hypromellose, iron oxide black and polyethylene glycol.
Clinical Pharmacology Section
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Irbesartan
Angiotensin II is a potent vasoconstrictor formed from angiotensin I in a
reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II).
Angiotensin II is the principal pressor agent of the RAS and also stimulates
aldosterone synthesis and secretion by adrenal cortex, cardiac contraction,
renal resorption of sodium, activity of the sympathetic nervous system, and
smooth muscle cell growth. Irbesartan blocks the vasoconstrictor and
aldosterone-secreting effects of angiotensin II by selectively binding to the
AT1 angiotensin II receptor. There is also an AT2 receptor in many tissues,
but it is not involved in cardiovascular homeostasis.
Irbesartan is a specific competitive antagonist of AT1 receptors with a much
greater affinity (more than 8500-fold) for the AT1 receptor than for the AT2
receptor, and no agonist activity.
Blockade of the AT1 receptor removes the negative feedback of angiotensin II
on renin secretion, but the resulting increased plasma renin activity and
circulating angiotensin II do not overcome the effects of irbesartan on blood
pressure.
Irbesartan does not inhibit ACE or renin or affect other hormone receptors or
ion channels known to be involved in the cardiovascular regulation of blood
pressure and sodium homeostasis. Because irbesartan does not inhibit ACE, it
does not affect the response to bradykinin; whether this has clinical
relevance is not known.
Hydrochlorothiazide
Hydrochlorothiazide is a thiazide diuretic. Thiazides affect the renal tubular
mechanisms of electrolyte reabsorption, directly increasing excretion of
sodium and chloride in approximately equivalent amounts. Indirectly, the
diuretic action of hydrochlorothiazide reduces plasma volume, with consequent
increases in plasma renin activity, increases in aldosterone secretion,
increases in urinary potassium loss, and decreases in serum potassium. The
renin-aldosterone link is mediated by angiotensin II, so coadministration of
an angiotensin II receptor antagonist tends to reverse the potassium loss
associated with these diuretics.
The mechanism of the antihypertensive effect of thiazides is not fully understood.
12.2 Pharmacodynamics
Irbesartan
In healthy subjects, single oral irbesartan doses of up to 300 mg produced
dose-dependent inhibition of the pressor effect of angiotensin II infusions.
Inhibition was complete (100%) 4 hours following oral doses of 150 mg or 300
mg and partial inhibition was sustained for 24 hours (60% and 40% at 300 mg
and 150 mg, respectively).
In hypertensive patients, angiotensin II receptor inhibition following chronic
administration of irbesartan causes a 1.5-fold to 2-fold rise in angiotensin
II plasma concentration and a 2-fold to 3-fold increase in plasma renin
levels. Aldosterone plasma concentrations generally decline following
irbesartan administration, but serum potassium levels are not significantly
affected at recommended doses.
In hypertensive patients, chronic oral doses of irbesartan (up to 300 mg) had
no effect on glomerular filtration rate, renal plasma flow or filtration
fraction. In multiple dose studies in hypertensive patients, there were no
clinically important effects on fasting triglycerides, total cholesterol, HDL-
cholesterol, or fasting glucose concentrations. There was no effect on serum
uric acid during chronic oral administration and no uricosuric effect.
Hydrochlorothiazide
After oral administration of hydrochlorothiazide, diuresis begins within 2
hours, peaks in about 4 hours and lasts about 6 to 12 hours.
Drug Interactions
Hydrochlorothiazide
Alcohol, barbiturates, or narcotics: Potentiation of orthostatic hypotension may occur.
Skeletal muscle relaxants: Possible increased responsiveness to muscle relaxants such as curare derivatives.
Corticosteroids, ACTH: Intensified electrolyte depletion, particularly hypokalemia.
Pressor amines (e.g., norepinephrine): Possible decreased response to pressor amines but not sufficient to preclude their use.
12.3 Pharmacokinetics
Irbesartan
Irbesartan is an orally active agent that does not require biotransformation
into an active form. The oral absorption of irbesartan is rapid and complete
with an average absolute bioavailability of 60% to 80%. Following oral
administration of irbesartan, peak plasma concentrations of irbesartan are
attained at 1.5 to 2 hours after dosing. Food does not affect the
bioavailability of irbesartan.
Irbesartan exhibits linear pharmacokinetics over the therapeutic dose range.
The terminal elimination half-life of irbesartan averaged 11 to 15 hours.
Steady-state concentrations are achieved within 3 days. Limited accumulation
of irbesartan (<20%) is observed in plasma upon repeated once-daily dosing.
Hydrochlorothiazide
When plasma levels have been followed for at least 24 hours, the plasma half-
life has been observed to vary between 5.6 and 14.8 hours.
Metabolism and Elimination
Irbesartan
Irbesartan is metabolized via glucuronide conjugation and oxidation. Following
oral or intravenous administration of 14C-labeled irbesartan, more than 80% of
the circulating plasma radioactivity is attributable to unchanged irbesartan.
The primary circulating metabolite is the inactive irbesartan glucuronide
conjugate (approximately 6%). The remaining oxidative metabolites do not add
appreciably to irbesartan's pharmacologic activity.
Irbesartan and its metabolites are excreted by both biliary and renal routes.
Following either oral or intravenous administration of 14C-labeled irbesartan,
about 20% of radioactivity is recovered in the urine and the remainder in the
feces, as irbesartan or irbesartan glucuronide.
In vitro studies of irbesartan oxidation by cytochrome P450 isoenzymes
indicated irbesartan was oxidized primarily by 2C9; metabolism by 3A4 was
negligible. Irbesartan was neither metabolized by, nor did it substantially
induce or inhibit, isoenzymes commonly associated with drug metabolism (1A1,
1A2, 2A6, 2B6, 2D6, 2E1). There was no induction or inhibition of 3A4.
Hydrochlorothiazide
Hydrochlorothiazide is not metabolized but is eliminated rapidly by the
kidney. At least 61% of the oral dose is eliminated unchanged within 24 hours.
Distribution
Irbesartan
Irbesartan is 90% bound to serum proteins (primarily albumin and α1-acid
glycoprotein) with negligible binding to cellular components of blood. The
average volume of distribution is 53 to 93 liters. Total plasma and renal
clearances are in the range of 157 to 176 mL/min and 3.0 to 3.5 mL/min,
respectively. With repetitive dosing, irbesartan accumulates to no clinically
relevant extent.
Studies in animals indicate that radiolabeled irbesartan weakly crosses the
blood-brain barrier and placenta. Irbesartan is excreted in the milk of
lactating rats.
Hydrochlorothiazide
Hydrochlorothiazide crosses the placental but not the blood-brain barrier and
is excreted in breast milk.
Specific Populations
Pediatric
Irbesartan and hydrochlorothiazide pharmacokinetics have not been investigated
in patients <18 years of age.
Gender
No gender-related differences in pharmacokinetics were observed in healthy
elderly (age 65 to 80 years) or in healthy young (age 18 to 40 years)
subjects. In studies of hypertensive patients, there was no gender difference
in half-life or accumulation, but somewhat higher plasma concentrations of
irbesartan were observed in females (11% to 44%). No gender-related dosage
adjustment is necessary.
Geriatric
In elderly subjects (age 65 to 80 years), irbesartan elimination half-life was
not significantly altered, but AUC and Cmax values were about 20% to 50%
greater than those of young subjects (age 18 to 40 years). No dosage
adjustment is necessary in the elderly.
Race
In healthy black subjects, irbesartan AUC values were approximately 25%
greater than whites; there were no differences in Cmax values.
Renal insufficiency
The pharmacokinetics of irbesartan were not altered in patients with renal
impairment or in patients on hemodialysis. Irbesartan is not removed by
hemodialysis. No dosage adjustment is necessary in patients with mild to
severe renal impairment unless a patient with renal impairment is also volume
depleted [see Warnings and Precautions (5.2)].
Hepatic insufficiency
The pharmacokinetics of irbesartan following repeated oral administration were
not significantly affected in patients with mild to moderate cirrhosis of the
liver. No dosage adjustment is necessary in patients with hepatic
insufficiency.
Drug-Drug Interactions
No significant drug-drug pharmacokinetic (or pharmacodynamic) interactions
have been found in interaction studies with hydrochlorothiazide, digoxin,
warfarin, and nifedipine.
In vitro studies show significant inhibition of the formation of oxidized irbesartan metabolites with the known cytochrome CYP2C9 substrates/inhibitors sulphenazole, tolbutamide and nifedipine. However, in clinical studies the consequences of concomitant irbesartan on the pharmacodynamics of warfarin were negligible. Concomitant nifedipine or hydrochlorothiazide had no effect on irbesartan pharmacokinetics. Based on in vitro data, no interaction would be expected with drugs whose metabolism is dependent upon cytochrome P450 isoenzymes 1A1, 1A2, 2A6, 2B6, 2D6, 2E1, or 3A4.
In separate studies of patients receiving maintenance doses of warfarin, hydrochlorothiazide, or digoxin, irbesartan administration for 7 days had no effect on the pharmacodynamics of warfarin (prothrombin time) or the pharmacokinetics of digoxin. The pharmacokinetics of irbesartan were not affected by coadministration of nifedipine or hydrochlorothiazide.
Nonclinical Toxicology Section
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Irbesartan and Hydrochlorothiazide
No carcinogenicity studies have been conducted with the irbesartan and
hydrochlorothiazide combination.
Irbesartan and hydrochlorothiazide was not mutagenic in standard in vitro
tests (Ames microbial test and Chinese hamster mammalian-cell forward gene-
mutation assay). Irbesartan and hydrochlorothiazide was negative in tests for
induction of chromosomal aberrations (in vitro–human lymphocyte assay; in vivo
– mouse micronucleus study).
The combination of irbesartan and hydrochlorothiazide has not been evaluated
in definitive studies of fertility.
Irbesartan
No evidence of carcinogenicity was observed when irbesartan was administered
at doses of up to 500/1000 mg/kg/day (males/females, respectively) in rats and
1000 mg/kg/day in mice for up to 2 years. For male and female rats, 500
mg/kg/day provided an average systemic exposure to irbesartan (AUC0-24 hours,
bound plus unbound) about 3 and 11 times, respectively, the average systemic
exposure in humans receiving the maximum recommended dose (MRHD) of 300 mg
irbesartan/day, whereas 1000 mg/kg/day (administered to females only) provided
an average systemic exposure about 21 times that reported for humans at the
MRHD. For male and female mice, 1000 mg/kg/day provided an exposure to
irbesartan about 3 and 5 times, respectively, the human exposure at 300
mg/day.
Irbesartan was not mutagenic in a battery of in vitro tests (Ames microbial
test, rat hepatocyte DNA repair test, V79 mammalian-cell forward gene-mutation
assay). Irbesartan was negative in several tests for induction of chromosomal
aberrations (in vitro–human lymphocyte assay; in vivo–mouse micronucleus
study).
Irbesartan had no adverse effects on fertility or mating of male or female
rats at oral doses ≤650 mg/kg/day, the highest dose providing a systemic
exposure to irbesartan (AUC0-24 hours, bound plus unbound) about 5 times that
found in humans receiving the MRHD of 300 mg/day.
Hydrochlorothiazide
Two-year feeding studies in mice and rats conducted under the auspices of the
National Toxicology Program (NTP) uncovered no evidence of a carcinogenic
potential of hydrochlorothiazide in female mice (at doses of up to
approximately 600 mg/kg/day) or in male and female rats (at doses of up to
approximately 100 mg/kg/day). The NTP, however, found equivocal evidence for
hepatocarcinogenicity in male mice.
Hydrochlorothiazide was not genotoxic in vitro in the Ames mutagenicity assay
of Salmonella typhimurium strains TA 98, TA 100, TA 1535, TA 1537, and TA 1538
and in the Chinese Hamster Ovary (CHO) test for chromosomal aberrations, or in
vivo in assays using mouse germinal cell chromosomes, Chinese hamster bone
marrow chromosomes, and the Drosophila sex-linked recessive lethal trait gene.
Positive test results were obtained only in the in vitro CHO Sister Chromatid
Exchange (clastogenicity) and in the Mouse Lymphoma Cell (mutagenicity)
assays, using concentrations of hydrochlorothiazide from 43 to 1300 μg/mL, and
in the Aspergillus nidulans non-disjunction assay at an unspecified
concentration.
Hydrochlorothiazide had no adverse effects on the fertility of mice and rats
of either sex in studies wherein these species were exposed, via their diet,
to doses of up to 100 and 4 mg/kg, respectively, prior to mating and
throughout gestation.
Clinical Studies Section
14. CLINICAL STUDIES
14.1 Irbesartan Monotherapy
The antihypertensive effects of irbesartan were examined in 7 major placebo-
controlled, 8 to 12-week trials in patients with baseline diastolic blood
pressures of 95 to 110 mmHg. Doses of 1 to 900 mg were included in these
trials in order to fully explore the dose-range of irbesartan. These studies
allowed a comparison of once or twice-daily regimens at 150 mg/day,
comparisons of peak and trough effects, and comparisons of response by gender,
age, and race. Two of the 7 placebo-controlled trials identified above and 2
additional placebo-controlled studies examined the antihypertensive effects of
irbesartan and hydrochlorothiazide in combination.
The 7 studies of irbesartan monotherapy included a total of 1915 patients
randomized to irbesartan (1 to 900 mg) and 611 patients randomized to placebo.
Once-daily doses of 150 to 300 mg provided statistically and clinically
significant decreases in systolic and diastolic blood pressure with trough
(24-hour post dose) effects after 6 to 12 weeks of treatment compared to
placebo, of about 8 to 10/5 to 6 mmHg and 8 to 12/5 to 8 mmHg, respectively.
No further increase in effect was seen at dosages greater than 300 mg. The
dose-response relationships for effects on systolic and diastolic pressure are
shown in Figures 3 and 4.
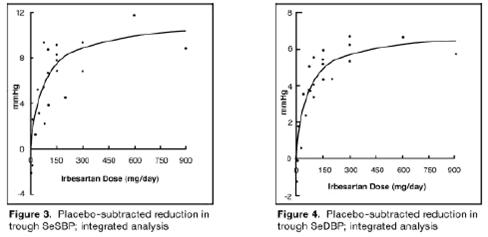
Once–daily administration of therapeutic doses of irbesartan gave peak effects
at around 3 to 6 hours and, in one continuous ambulatory blood pressure
monitoring study, again around 14 hours. This was seen with both once-daily
and twice–daily dosing. Trough-to-peak ratios for systolic and diastolic
response were generally between 60% and 70%. In a continuous ambulatory blood
pressure monitoring study, once-daily dosing with 150 mg gave trough and mean
24-hour responses similar to those observed in patients receiving twice-daily
dosing at the same total daily dose.
Analysis of age, gender, and race subgroups of patients showed that men and
women, and patients over and under 65 years of age, had generally similar
responses. Irbesartan was effective in reducing blood pressure regardless of
race, although the effect was somewhat less in blacks (usually a low-renin
population). Black patients typically show an improved response with the
addition of a low dose diuretic (e.g., 12.5 mg hydrochlorothiazide).
The effect of irbesartan is apparent after the first dose and is close to the
full observed effect at 2 weeks. At the end of the 8-week exposure, about 2/3
of the antihypertensive effect was still present 1 week after the last dose.
Rebound hypertension was not observed. There was essentially no change in
average heart rate in irbesartan-treated patients in controlled trials.
14.2 Irbesartan and Hydrochlorothiazide
The antihypertensive effects of Irbesartan and Hydrochlorothiazide Tablets
were examined in 4 placebo-controlled studies in patients with mild-moderate
hypertension (mean seated diastolic blood pressure [SeDBP] between 90 and 110
mmHg), one study in patients with moderate hypertension (mean seated systolic
blood pressure [SeSBP] 160 to 179 mmHg or SeDBP 100 to 109 mmHg), and one
study in patients with severe hypertension (mean SeDBP ≥110 mmHg) of 8 to 12
weeks. These trials included 3149 patients randomized to fixed doses of
irbesartan (37.5 to 300 mg) and concomitant hydrochlorothiazide (6.25 to 25
mg).
Study I was a factorial study that compared all combinations of irbesartan
(37.5 mg, 100 mg, and 300 mg or placebo) and hydrochlorothiazide (6.25 mg,
12.5 mg, and 25 mg or placebo).
Study II compared the Irbesartan and hydrochlorothiazide combinations of 75
mg/12.5 mg and 150 mg/12.5 mg to their individual components and placebo.
Study III investigated the ambulatory blood pressure responses to Irbesartan
and hydrochlorothiazide (75 mg/12.5 mg and 150 mg/12.5 mg) and placebo after 8
weeks of dosing.
Study IV investigated the effects of the addition of irbesartan (75 or 150 mg)
in patients not controlled (SeDBP 93 to 120 mmHg) on hydrochlorothiazide (25
mg) alone. In Studies Ito III, the addition of irbesartan 150 to 300 mg to
hydrochlorothiazide doses of 6.25, 12.5, or 25 mg produced further dose-
related reductions in blood pressure at trough of 8 to 10 mmHg/3 to 6 mmHg,
similar to those achieved with the same monotherapy dose of irbesartan. The
addition of hydrochlorothiazide to irbesartan produced further dose-related
reductions in blood pressure at trough (24 hours post dose) of 5 to 6 /2 to 3
mmHg (12.5 mg) and 7 to 11/4 to 5 mmHg (25 mg), also similar to effects
achieved with hydrochlorothiazide alone. Once-daily dosing with 150 mg
irbesartan and 12.5 mg hydrochlorothiazide, 300 mg irbesartan and 12.5 mg
hydrochlorothiazide, or 300 mg irbesartan and 25 mg hydrochlorothiazide
produced mean placebo-adjusted blood pressure reductions at trough (24 hours
post dosing) of about 13 to 15/7 to 9 mmHg, 14/9 to 12 mmHg, and 19 to 21/11
to 12 mmHg, respectively. Peak effects occurred at 3 to 6 hours, with the
trough-to-peak ratios >65%.
In Study IV, the addition of irbesartan (75to 150 mg) gave an additive effect
(systolic/diastolic) at trough (24 hours post dosing) of 11/7 mmHg.
Initial Therapy
Studies V and VI had no placebo group, so effects described below are not all
attributable to irbesartan or HCTZ.
Study V was conducted in patients with a mean baseline blood pressure of
162/98 mmHg and compared the change from baseline in SeSBP at 8 weeks between
the combination group (irbesartan and HCTZ 150 mg/12.5 mg), to irbesartan (150
mg) and to HCTZ (12.5 mg). These initial study regimens were increased at 2
weeks to Irbesartan and Hydrochlorothiazide Tablets 300 mg/25 mg, irbesartan
300 mg, or to HCTZ 25 mg, respectively.
Mean reductions from baseline for SeDBP and SeSBP at trough were 14.6 mmHg and
27.1 mmHg for patients treated with Irbesartan and Hydrochlorothiazide
Tablets, 11.6 mmHg and 22.1 mmHg for patients treated with irbesartan, and 7.3
mmHg and 15.7 mmHg for patients treated with HCTZ at 8 weeks, respectively.
For patients treated with Irbesartan and Hydrochlorothiazide Tablets, the mean
change from baseline in SeDBP was 3 mmHg lower (p=0.0013) and the mean change
from baseline in SeSBP was 5 mmHg lower (p=0.0016) compared to patients
treated with irbesartan, and 7.4 mmHg lower (p<0.0001) and 11.3 mmHg lower
(p<0.0001) compared to patients treated with HCTZ, respectively. Withdrawal
rates were 3.8% on irbesartan, 4.8% on HCTZ, and 6.7% on Irbesartan and
Hydrochlorothiazide Tablets.
Study VI was conducted in patients with a mean baseline blood pressure of
172/113 mmHg and compared trough SeDBP at 5 weeks between the combination
group (irbesartan and HCTZ 150 mg/12.5 mg) and irbesartan (150 mg). These
initial study regimens were increased at 1 week to Irbesartan and
Hydrochlorothiazide Tablets 300 mg/25 mg or to irbesartan 300 mg,
respectively.
At 5 weeks, mean reductions from baseline for SeDBP and SeSBP at trough were
24 mmHg and 30.8 mmHg for patients treated with Irbesartan and
Hydrochlorothiazide Tablets and 19.3 mmHg and 21.1 mmHg for patients treated
with irbesartan, respectively. The mean SeDBP was 4.7 mmHg lower (p<0.0001)
and the mean SeSBP was 9.7 mmHg lower (p<0.0001) in the group treated with
Irbesartan and Hydrochlorothiazide Tablets than in the group treated with
irbesartan. Patients treated with Irbesartan and Hydrochlorothiazide Tablets
achieved more rapid blood pressure control with significantly lower SeDBP and
SeSBP and greater blood pressure control at every assessment (Week 1, Week 3,
Week 5, and Week 7). Maximum effects were seen at Week 7.
Withdrawal rates were 2.2% on irbesartan and 2.1% on Irbesartan and
Hydrochlorothiazide Tablets.
In Studies I to VI there was no difference in response for men and women or in
patients over or under 65 years of age. Black patients had a larger response
to hydrochlorothiazide than non-black patients and a smaller response to
irbesartan. The overall response to the combination was similar for black and
non-black patients.
How Supplied Section
16. HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Irbesartan and Hydrochlorothiazide Tablets USP are supplied as follows:
Irbesartan and Hydrochlorothiazide Tablets USP 150 mg/12.5 mg:
Peach coloured mottled, oval shaped, biconvex, uncoated tablets debossed with
“L180” on one side and plain on other side.
NDC 62332-051-30 bottle of 30 tablets.
NDC 62332-051-90 bottle of 90 tablets.
NDC 62332-051-91 bottle of 1000 tablets.
NDC 62332-051-10 carton of 10 blisters of 10 tablets.
Irbesartan and Hydrochlorothiazide Tablets USP 300 mg/12.5 mg:
Peach coloured mottled, oval shaped, biconvex, uncoated tablets debossed with
“L181” on one side and plain on other side.
NDC 62332-052-30 bottle of 30 tablets.
NDC 62332-052-90 bottle of 90 tablets.
NDC 62332-052-91 bottle of 1000 tablets.
NDC 62332-052-10 carton of 10 blisters of 10 tablets.
Irbesartan and Hydrochlorothiazide Tablets USP 300 mg/25 mg:
Pinkish brown, oval shaped, biconvex, film coated tablets, debossed with
“L182” on one side and plain on other side.
NDC 62332-053-30 bottle of 30 tablets.
NDC 62332-053-90 bottle of 90 tablets.
NDC 62332-053-91 bottle of 1000 tablets.
NDC 62332-053-10 carton of 10 blisters of 10 tablets.
16.2 Storage
Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
