Numb 520
350e4d5e-dac7-ae4e-e063-6394a90a76b4
HUMAN OTC DRUG LABEL
May 27, 2025
Ebanel Laboratories, Inc
DUNS: 079352161
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Lidocaine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (17)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
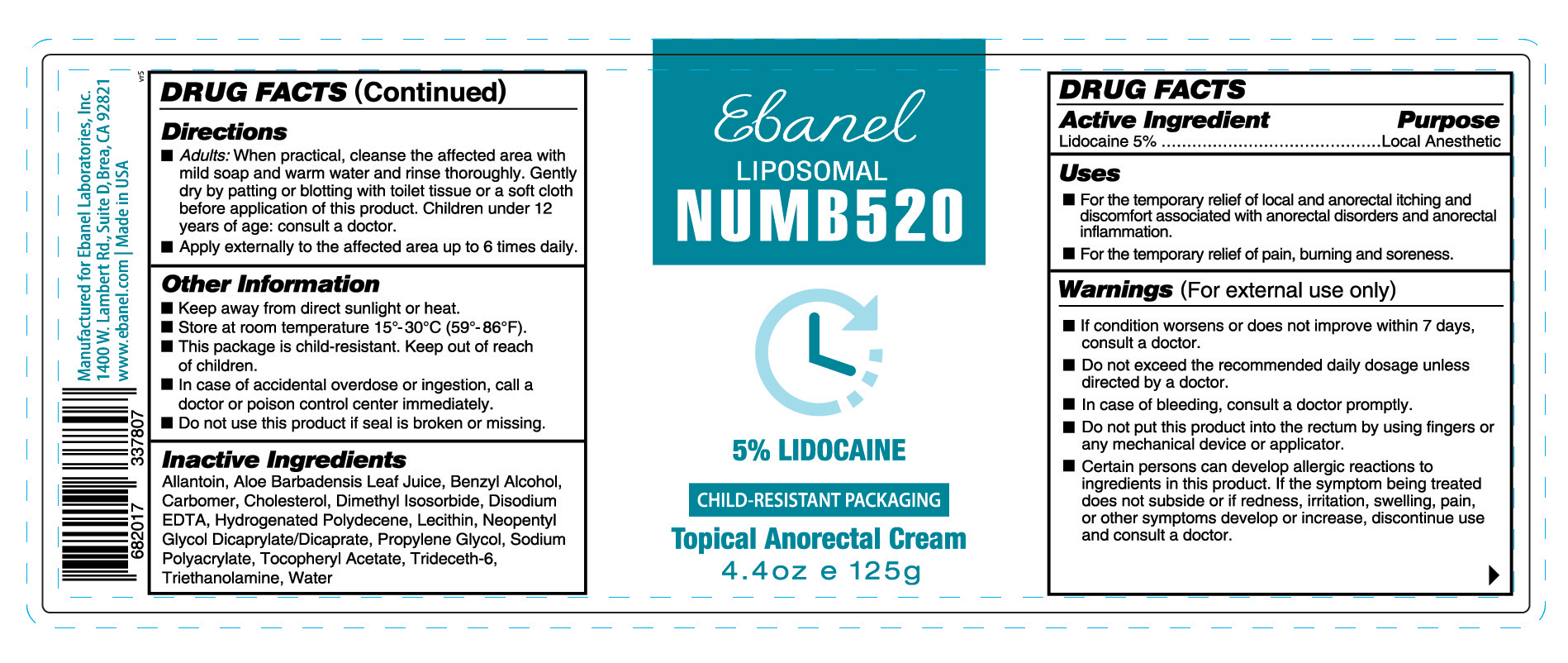 NUMB520
NUMB520
5% LIDOCAINE
CHILD-RESISTANT PACKAGING
Topical Anorectal Cream
4.4 oz e 125 g
INDICATIONS & USAGE SECTION
Uses
■ For the temporary relief of local and anorectal itching and discomfort associated with anorectal disorders and anorectal inflammation.
■ For the temporary relief of pain, burning and soreness.
WARNINGS SECTION
Warnings (For external use only)
■ If condition worsens or does not improve within 7 days, consult a doctor.
■ Do not exceed the recommended daily dosage unless directed by a doctor.
■ In case of bleeding, consult a doctor promptly.
■ Do not put this product into the rectum by using fingers or any mechanical device or applicator.
■ Certain persons can develop allergic reactions to ingredients in this product. If the symptom being treated does not subside or if redness, irritation, swelling, pain, or other symptoms develop or increase, discontinue use and consult a doctor.
OTC - ACTIVE INGREDIENT SECTION
Active Ingredient Purpose
Lidocaine 5% ………………………………………….Local Anesthetic
DOSAGE & ADMINISTRATION SECTION
Directions
■ Adults: When practical, cleanse the affected area with mild soap and warm water and rinse thoroughly. Gently dry by patting or blotting with toilet tissue or a soft cloth before application of this product. Children under 12 years of age: consult a doctor.
■ Apply externally to the affected area up to 6 times daily.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Other Information
■ Keep away from direct sunlight or heat.
■ Store at room temperature 15°-30°C (59°-86°F).
■ This package is child-resistant. Keep out of reach of children.
■ In case of accidental overdose or ingestion, call a doctor or poison control center immediately.
■ Do not use this product if seal is broken or missing.
INACTIVE INGREDIENT SECTION
Inactive Ingredients
Allantoin, Aloe Barbadensis Leaf Juice, Benzyl Alcohol, Carbomer, Cholesterol, Dimethyl Isosorbide, Disodium EDTA, Hydrogenated Polydecene, Lecithin, Neopentyl Glycol Dicaprylate/Dicaprate, Propylene Glycol, Sodium Polyacrylate, Tocopheryl Acetate, Trideceth-6, Triethanolamine, Water
OTC - PURPOSE SECTION
Purpose………………………………………….Local Anesthetic
