DEXTROSE
These highlights do not include all the information needed to use safely and effectively. See full prescribing information for . Initial U.S. Approval: 1940
36e9478c-5df0-4b47-b97d-de3626d7cb29
HUMAN PRESCRIPTION DRUG LABEL
Aug 12, 2020
Baxter Healthcare Corporation
DUNS: 005083209
Products 10
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
dextrose monohydrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
dextrose monohydrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
dextrose monohydrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
dextrose monohydrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
dextrose monohydrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
dextrose monohydrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
dextrose monohydrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
dextrose monohydrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
dextrose monohydrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
dextrose monohydrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL

Container Label
Baxter
NDC 0338-9651-75
UE0086DG
50mL
Viaflo
5% Dextrose Injection USP
pH 4.0 (3.2-6.5)
Osmolarity 252 mOsmol/L (calc)
Sterile non pyrogenic
Single dose container
Each 50 mL contains 2.5 g Dextrose Hydrous USP
Read Package Leaflet for full information
Additives may be incompatible Dosage Intravenously
as directed by a physician Cautions Must not
be used in series connections Do not administer
simultaneously with blood Do not use unless solution
is clear Rx Only Store unit in moisture barrier
overwrap at room temperature (25°C/77°F) until
ready to use Avoid excessive heat See insert
VIAFLO is not made from natural rubber latex, DEHP,
or PVC
7 Symbol
Baxter and Viaflo are trademarks of Baxter
International Inc. For product information
1-800-933-0303Baxter Healthcare Corporation
Deerfield IL 60015 USA Made in Spain
UN-35-04-151 1
Lot
Expiry
(01) 00303389651758

Carton Label
UE0086DG
75 x 50 mL
(** ≈**** 6 kg)**
5% Dextrose Injection, USP
Formula per 50 mL
****Dextrose Hydrous 2.5 g
Water for Injection
Osmolarity 252 mOsmol/L (calc)
pH 4.0 (3.5-6.5)
IV administration.
Read package insert before use.
****Keep out of reach and sight of children.
Do not connect partially used drugs.
Baxter Healthcare Corporation
Deerfield IL 60015 USA
Made in Spain
Viaflo
P333E750
?? - 0001
Lot: ????????
Expiry: YYYY/MM
(01)50303389651753(17)YYMM00(10)????????
UE0086DG
75 x 50 mL
**( ≈**6 kg)
5% Dextrose
Injection, USP
Viaflo
2D Barcode
Lot:
????????
Expiry:
YYYY/MM
(01)50303389651753(17)YYMM00(10)????????

Container Label
Baxter
NDC 0338-9649-75
UE0086G
50mL
Viaflo
5% Dextrose Injection USP
pH 4.0 (3.2-6.5)
Osmolarity 252 mOsmol/L (calc)
Sterile non pyrogenic
Single dose container
Each 50 mL contains 2.5 g Dextrose Hydrous USP
Read Package Leaflet for full information
Additives may be incompatible Dosage Intravenously
as directed by a physician Cautions Must not
be used in series connections Do not administer
simultaneously with blood Do not use unless solution
is clear Rx Only Store unit in moisture barrier
overwrap at room temperature (25°C/77°F) until
ready to use Avoid excessive heat See insert
VIAFLO is not made from natural rubber latex, DEHP,
or PVC
7 Symbol
Baxter and Viaflo are trademarks of Baxter
International Inc
For product information 1-800-933-0303
Baxter Healthcare Corporation
Deerfield IL 60015 USA Made in Spain
UN-35-04-348 1
Lot
Expiry
(01)00303389649755

Carton Label
UE0086G
75 x 50 mL
(** ≈**** 6 kg)**
5% Dextrose Injection, USP
Formula per 50 mL
****Dextrose Hydrous 2.5 g
Water for Injection
Osmolarity 252 mOsmol/L (calc)
pH 4.0 (3.5-6.5)
IV administration.
Read package insert before use.
****Keep out of reach and sight of children.
Do not connect partially used drugs.
Baxter Healthcare Corporation
Deerfield IL 60015 USA
Made in Spain
Viaflo
P333E746
?? - 0001
Lot: ????????
Expiry: YYYY/MM
(01)50303389649750(17)YYMM00(10)????????
UE0086G
75 x 50 mL
**( ≈**6 kg)
5% Dextrose
Injection, USP
Viaflo
2D Barcode
Lot:
????????
Expiry:
YYYY/MM
(01) 50303389649750(17)YYMM00(10)????????
****
Container Label
Baxter
NDC 0338-9655-60
UE0087DG
100mL
Viaflo
5% Dextrose Injection USP
pH 4.0 (3.2-6.5)
Osmolarity 252 mOsmol/L (calc)
Sterile non pyrogenic
Single dose container
Each 100 mL contains 5 g Dextrose Hydrous USP
Read Package Leaflet for full information
Additives may be incompatible Dosage Intravenously
as directed by a physician Cautions Must not
be used in series connections Do not administer
simultaneously with blood Do not use unless solution
is clear Rx Only Store unit in moisture barrier
overwrap at room temperature (25°C/77°F) until ready
to use Avoid excessive heat See insert
VIAFLO is not made from natural rubber latex, DEHP,
or PVC
7 Symbol
Baxter and Viaflo are trademarks of
Baxter International Inc
For product information 1-800-933-0303
Baxter Healthcare Corporation
Deerfield IL 60015 USA Made in Spain
UN-35-04-152 1
Lot
Expiry
(01)00303389655602

Carton Label
UE0087DG
60 x 100 mL
(** ≈**** 8 kg)**
5% Dextrose Injection, USP
Formula per 100 mL
****Dextrose Hydrous 5 g
Water for Injection
Osmolarity 252 mOsmol/L (calc)
pH 4.0 (3.5-6.5)
IV administration.
Read package insert before use.
****Keep out of reach and sight of children.
Do not connect partially used drugs.
Baxter Healthcare Corporation
Deerfield IL 60015 USA
Made in Spain
Viaflo
P333E751
?? - 0001
Lot: ????????
Expiry: YYYY/MM
(01)50303389655607(17)YYMM00(10)????????
UE0087DG
60 x 100 mL
( ≈** 8 kg)**
5% Dextrose
Injection, USP
Viaflo
2D Barcode
Lot:
????????
Expiry:
YYYY/MM
(01)50303389655607(17)YYMM00(10)????????

Container Label
Baxter
NDC 0338-9653-60
UE0087G
100mL
Viaflo
5% Dextrose Injection USP
pH 4.0 (3.2-6.5)
Osmolarity 252 mOsmol/L (calc)
Sterile non pyrogenic
Single dose container
Each 100 mL contains 5 g Dextrose Hydrous USP
Read Package Leaflet for full information
Additives may be incompatible Dosage Intravenously
as directed by a physician Cautions Must not
be used in series connections Do not administer
simultaneously with blood Do not use unless solution
is clear Rx Only Store unit in moisture barrier
overwrap at room temperature (25°C/77°F) until ready
to use Avoid excessive heat See insert
VIAFLO is not made from natural rubber latex, DEHP,
or PVC
7 Symbol
Baxter and Viaflo are trademarks of
Baxter International Inc
For product information 1-800-933-0303
Baxter Healthcare Corporation
Deerfield IL 60015 USA Made in Spain
UN-35-04-349 1
Lot
Expiry
(01)00303389653608

Carton Label
UE0087G
60 x 100 mL
(** ≈**** 8 kg)**
5% Dextrose Injection, USP
Formula per 100 mL
****Dextrose Hydrous 5 g
Water for Injection
Osmolarity 252 mOsmol/L (calc)
pH 4.0 (3.5-6.5)
IV administration.
Read package insert before use.
****Keep out of reach and sight of children.
Do not connect partially used drugs.
Baxter Healthcare Corporation
Deerfield IL 60015 USA
Made in Spain
Viaflo
P333E747
?? - 0001
Lot: ????????
Expiry: YYYY/MM
(01)50303389653603(17)YYMM00(10)????????
UE0087G
60 x 100 mL
**( ≈**8 kg)
5% Dextrose
Injection, USP
Viaflo
2D Barcode
Lot:
????????
Expiry:
YYYY/MM
(01)50303389653603(17)YYMM00(10)????????

Baxter
Viaflo
5% Dextrose
Injection USP
UE0062D
0338-0062-30
250 mL
Sterile non pyrogenic
Single dose container
pH 4.0 (3.2-6.5)
Osmolarity 252 mOsm/L (calc)
Each 100 mL contains 5 g Dextrose Hydrous USP Additives may be incompatible
Consult with pharmacist if available when introducing additives Use aseptic
technique Mix thoroughly Do not store Dosage intravenously as directed by a
physician See directions Cautions Squeeze and inspect inner bag which
maintains
product sterility Discard if leaks are found Must not be used in series
connections
Do not administer simultaneously with blood Do not use unless solution is
clear
Rx Only Store unit in moisture barrier overwrap at room temperature
(25°C/77°F)
until ready to use Avoid excessive heat See insert VIAFLO is not made from
natural
rubber latex, DEHP, or PVC
Baxter and Viaflo are trademarks of Baxter International Inc.
For product information
1-800-933-0303
Baxter Healthcare Corporation
Deerfield IL 60015 USA
Made in Spain
BAR CODE
Lot
UN-
Expiry
50
100
150
200

Baxter
Viaflo
5% Dextrose
Injection USP
UE0062
0338-0074-30
250 mL
Sterile non pyrogenic
Single dose container
pH 4.0 (3.2-6.5)
Osmolarity 252 mOsm/L (calc)
Each 100 mL contains 5 g Dextrose Hydrous USP Additives may be incompatible
Consult with pharmacist if available when introducing additives Use aseptic
technique Mix thoroughly Do not store Dosage intravenously as directed by a
physician See directions Cautions Squeeze and inspect inner bag which
maintains
product sterility Discard if leaks are found Must not be used in series
connections
Do not administer simultaneously with blood Do not use unless solution is
clear
Rx Only Store unit in moisture barrier overwrap at room temperature
(25°C/77°F)
until ready to use Avoid excessive heat See insert VIAFLO is not made from
natural
rubber latex, DEHP, or PVC
Baxter and Viaflo are trademarks of Baxter International Inc.
For product information
1-800-933-0303
Baxter Healthcare Corporation
Deerfield IL 60015 USA
Made in Spain
BAR CODE
Lot
UN-
Expiry
50
100
150
200
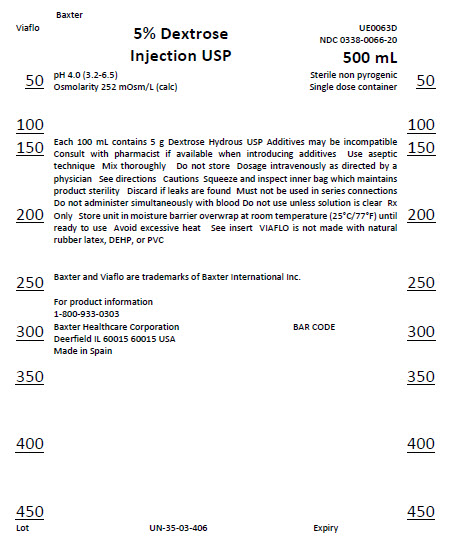
Baxter
Viaflo
5% Dextrose
Injection USP
UE0063D
0338-0066-20
500 mL
Sterile non pyrogenic
Single dose container
pH 4.0 (3.2-6.5)
Osmolarity 252 mOsm/L (calc)
Each 100 mL contains 5 g Dextrose Hydrous USP Additives may be incompatible
Consult with pharmacist if available when introducing additives Use aseptic
technique Mix thoroughly Do not store Dosage intravenously as directed by a
physician See directions Cautions Squeeze and inspect inner bag which
maintains
product sterility Discard if leaks are found Must not be used in series
connections
Do not administer simultaneously with blood Do not use unless solution is
clear
Rx Only Store unit in moisture barrier overwrap at room temperature
(25°C/77°F)
until ready to use Avoid excessive heat See insert VIAFLO is not made from
natural
rubber latex, DEHP, or PVC
Baxter and Viaflo are trademarks of Baxter International Inc.
For product information
1-800-933-0303
Baxter Healthcare Corporation
Deerfield IL 60015 USA
Made in Spain
BAR CODE
Lot
UN-35-03-406
Expiry
50
100
150
200
250
300
350
400
450

Baxter
Viaflo
5% Dextrose
Injection USP
UE0063
0338-0078-20
500 mL
Sterile non pyrogenic
Single dose container
pH 4.0 (3.2-6.5)
Osmolarity 252 mOsm/L (calc)
Each 100 mL contains 5 g Dextrose Hydrous USP Additives may be incompatible
Consult with pharmacist if available when introducing additives Use aseptic
technique Mix thoroughly Do not store Dosage intravenously as directed by a
physician See directions Cautions Squeeze and inspect inner bag which
maintains
product sterility Discard if leaks are found Must not be used in series
connections
Do not administer simultaneously with blood Do not use unless solution is
clear Rx
Only Store unit in moisture barrier overwrap at room temperature (25°C/77°F)
until
ready to use Avoid excessive heat See insert VIAFLO is not made from natural
rubber latex, DEHP, or PVC
Baxter and Viaflo are trademarks of Baxter International Inc.
For product information
1-800-933-0303
Baxter Healthcare Corporation
Deerfield IL 60015 USA
Made in Spain
BAR CODE
Lot
UN-35-03-405
Expiry
50
100
150
200
250
300
350
400
450
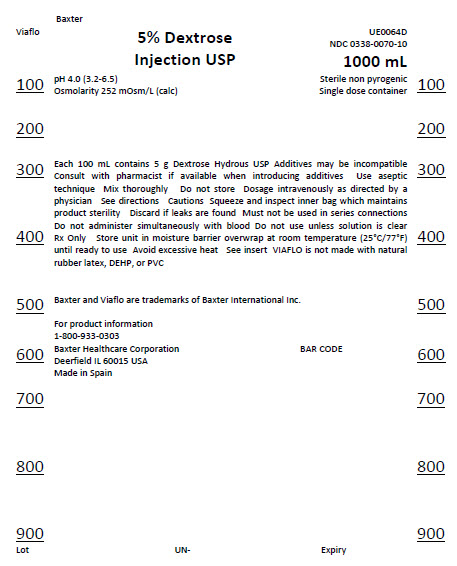
Baxter
Viaflo
5% Dextrose
Injection USP
UE0064D
0338-0070-10
1000 mL
Sterile non pyrogenic
Single dose container
pH 4.0 (3.2-6.5)
Osmolarity 252 mOsm/L (calc)
Each 100 mL contains 5 g Dextrose Hydrous USP Additives may be incompatible
Consult with pharmacist if available when introducing additives Use aseptic
technique Mix thoroughly Do not store Dosage intravenously as directed by a
physician See directions Cautions Squeeze and inspect inner bag which
maintains
product sterility Discard if leaks are found Must not be used in series
connections
Do not administer simultaneously with blood Do not use unless solution is
clear
Rx Only Store unit in moisture barrier overwrap at room temperature
(25°C/77°F)
until ready to use Avoid excessive heat See insert VIAFLO is not made from
natural
rubber latex, DEHP, or PVC
Baxter and Viaflo are trademarks of Baxter International Inc.
For product information
1-800-933-0303
Baxter Healthcare Corporation
Deerfield IL 60015 USA
Made in Spain
BAR CODE
Lot
UN-
Expiry
100
200
300
400
500
600
700
800
900

Baxter
Viaflo
5% Dextrose
Injection USP
UE0064
0338-0082-10
1000 mL
Sterile non pyrogenic
Single dose container
pH 4.0 (3.2-6.5)
Osmolarity 252 mOsm/L (calc)
Each 100 mL contains 5 g Dextrose Hydrous USP Additives may be incompatible
Consult with pharmacist if available when introducing additives Use aseptic
technique Mix thoroughly Do not store Dosage intravenously as directed by a
physician See directions Cautions Squeeze and inspect inner bag which
maintains
product sterility Discard if leaks are found Must not be used in series
connections
Do not administer simultaneously with blood Do not use unless solution is
clear
Rx Only Store unit in moisture barrier overwrap at room temperature
(25°C/77°F)
until ready to use Avoid excessive heat See insert VIAFLO is not made from
natural
rubber latex, DEHP, or PVC
Baxter and Viaflo are trademarks of Baxter International Inc.
For product information
1-800-933-0303
Baxter Healthcare Corporation
Deerfield IL 60015 USA
Made in Spain
BAR CODE
Lot
UN-
Expiry
100
200
300
400
500
600
700
800
900
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
•
Dextrose Injection is intended for intravenous use.
•
Peripheral administration of 5% dextrose is generally acceptable, however, consider central vein when administering more than 5% dextrose or with an osmolarity of at least 900 mOsm/L or when there is peripheral vein irritation, phlebitis, and/or associated pain [see Warnings and Precautions (5.3)].
•
Do not administer Dextrose Injection simultaneously with blood products through the same administration set because of the possibility of pseudoagglutination or hemolysis.
•
To prevent air embolism, use a non-vented infusion set or close the vent on a vented set, avoid multiple connections, do not connect flexible containers in series, fully evacuate residual gas in the container prior to administration, do not pressurize the flexible container to increase flow rates, and if administration is controlled by a pumping device, turn off pump before the container runs dry.
•
Prior to infusion, visually inspect the diluted dextrose solution for particulate matter. The solution should be clear and there should be no precipitates. Do not administer unless solution is clear and container is undamaged.
•
Use of a final filter is recommended during administration of parenteral solutions, where possible.
2.2 Recommended Dosage
The choice of dextrose concentration, rate and volume depends on the age, weight, clinical and metabolic conditions of the patient and concomitant therapy. Electrolyte supplementation may be indicated according to the clinical needs of the patient.
The administration rate should be governed, especially for premature infants with low birth weight, during the first few days of therapy, by the patient’s tolerance to dextrose.
Increase the infusion rate gradually as indicated by frequent monitoring of blood glucose concentrations [see Warnings and Precautions (5.1), Use in Specific Populations (8.4)].
2.3 Instructions for Use
To Open
•
Do not remove from overpouch until ready to use.
•
Tear overwrap down side at slit and remove solution container. Small amounts of moisture may be found on the solution container from water permeating from inside the container. The amount of permeated water is insufficient to affect the solution significantly. If larger amounts of water are found, the container should be checked for tears or leaks.
•
Visually inspect the container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually. Evaluate the following:
•
If the outlet port protector is damaged, detached, or not present, discard container.
•
Check to ensure the solution is clear and there are no precipitates. Discard if there is a color change and/or the appearance of precipitates, insoluble complexes or crystals.
•
Check for minute leaks by squeezing the inner bag firmly. If leaks are found, discard container.
Preparation for Administration
Suspend container from eyelet support.
Remove protector from outlet port at bottom of container.
Attach administration set. Refer to complete directions accompanying set.
To Add Medication
•
Additives may be incompatible. Complete information is not available. Do not use additives known or determined to be incompatible.
•
Consult with pharmacist, if available. If, in the informed judgment of the healthcare provider, it is deemed advisable to introduce additives, use aseptic technique.
•
When introducing additives, consult the instructions for use of the medication to be added and other relevant literature.
•
Before adding a substance or medication, verify that it is soluble and/or stable in Dextrose Injection and that the pH range of Dextrose Injection is appropriate.
To Add Medication Before Solution Administration
Prepare medication site.
Using syringe with 19 to 22 gauge needle, puncture resealable medication port and inject.
Mix solution and medication thoroughly. For high density medication such as potassium chloride, squeeze ports while ports are upright and mix thoroughly.
After addition, check to ensure the solution is clear and there are no precipitates. Discard if there is a color change and/or the appearance of precipitates, insoluble complexes or crystals.
To Add Medication During Solution Administration
Close clamp on the set.
Prepare medication site.
Using syringe with 19 to 22 gauge needle, puncture resealable medication port and inject.
Remove container from IV pole and/or turn to an upright position.
Evacuate both ports by squeezing them while container is in the upright position.
Mix solution and medication thoroughly.
After addition, check to ensure the solution is clear and there are no precipitates. Discard if there is a color change and/or the appearance of precipitates, insoluble complexes or crystals, do not use.
Return container to in-use position and continue administration.
Storage
•
Use promptly; do not store solutions containing additives.
•
Single-dose container.
•
Discard any unused portion.
•
Only for intravenous infusion. (2.1)
•
See full prescribing information for information on preparation, administration, dosing considerations and instructions for use. (2.1, 2.2, 2.3)
