Sovereign Silver First Aid Gel Topical Healing Homeopathic Medicine
b3a22f82-fc37-4426-bef2-15ae71b1b681
HUMAN OTC DRUG LABEL
May 22, 2025
Natural Immunogenics Corp.dba SOVEREIGN NATURALS
DUNS: 048744085
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Argentum Metallicum
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
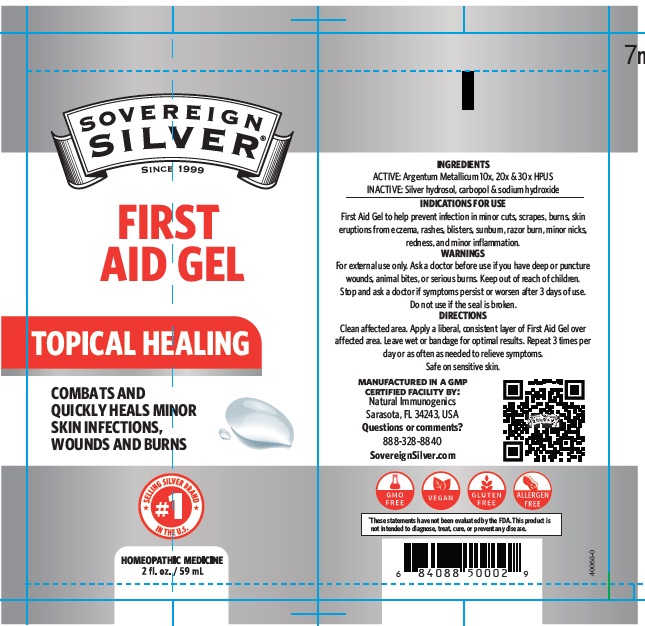
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Product label

INDICATIONS & USAGE SECTION
Uses
FIRST AID GEL TO HELP PREVENT INFECTION IN MINOR CUTS, SCRAPES, BURNS, SKIN ERUPTIONS FROM ECZEMA, RASHES, BLISTERS, SUNBURN, RAZOR BURN, MINOR NICKS, REDNESS AND MINOR INFLAMMATION.
OTC - ACTIVE INGREDIENT SECTION
Active Ingredient
ARGENTUM METALLICUM 10X, 20X, 30X HPUS
OTC - PURPOSE SECTION
Purpose
HOMEOPATHIC REMEDY FOR TOPICAL APPLICATIONS
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children.
WARNINGS SECTION
Warning
FOR EXTERNAL USE ONLY. ASK A DOCTOR BEFORE USE IF YOU HAVE DEEP OR PUNCTURE WOUNDS, ANIMAL BITES OR SERIOUS BURNS. KEEP OUT OF REACH OF CHILDREN. STOP AND ASK A DOCTOR IF SYMPTOMS PERSIST OR WORSEN AFTER 3 DAYS OF USE. DO NOT USE IF THE SEAL IS BROKEN.
DOSAGE & ADMINISTRATION SECTION
Directions
CLEAN AFFECTED AREA. APPLY A LIBERAL, CONSISTENT LAYER OF FIRST AID GEL OVER AFFECTED AREA. LEAVE WET OR BANDAGE FOR OPTIMAL RESULTS. REPEAT 3 TIMES PER DAY OR AS OFTEN AS NEEDED TO RELIEVE SYMPTOMS. SAFE ON SENSITIVE SKIN.
INACTIVE INGREDIENT SECTION
Inactive Ingredients
CARBOPOL, SILVER HYDROSOL AND SODIUM HYDROXIDE
