donepezil hydrochloride
These highlights do not include all the information needed to use DONEPEZIL HYDROCHLORIDE TABLETS safely and effectively. See full prescribing information for DONEPEZIL HYDROCHLORIDE TABLETS. DONEPEZIL HYDROCHLORIDE tablets, for oral useInitial U.S. Approval: 1996
48178cb4-4fee-47c0-88e9-a4f017f1f448
HUMAN PRESCRIPTION DRUG LABEL
Jun 30, 2025
Solco Healthcare US, LLC
DUNS: 828343017
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
donepezil hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
donepezil hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
**Principal Display Panel – 10 mg Bottle Label- Alternate Manufacturing
Site Prinston Laboratories**
NDC 43547-879-03 Rx only
Donepezil HCI****Tablets, USP
10 mg****30 Tablets
Solco****Healthcare U.S.

WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Anesthesia
Donepezil hydrochloride, as a cholinesterase inhibitor, is likely to exaggerate succinylcholine-type muscle relaxation during anesthesia.
5.2 Cardiovascular Conditions
Because of their pharmacological action, cholinesterase inhibitors may have vagotonic effects on the sinoatrial and atrioventricular nodes. This effect may manifest as bradycardia or heart block in patients both with and without known underlying cardiac conduction abnormalities. Syncopal episodes have been reported in association with the use of donepezil hydrochloride.
5.3 Nausea and Vomiting
Donepezil hydrochloride, as a predictable consequence of its pharmacological properties, has been shown to produce diarrhea, nausea, and vomiting. These effects, when they occur, appear more frequently with the 10 mg/day dose than with the 5 mg/day dose.
Although in most cases, these effects have been transient, sometimes lasting one to three weeks, and have resolved during continued use of donepezil hydrochloride, patients should be observed closely at the initiation of treatment and after dose increases.
5.4 Peptic Ulcer Disease and GI Bleeding
Through their primary action, cholinesterase inhibitors may be expected to increase gastric acid secretion due to increased cholinergic activity. Therefore, patients should be monitored closely for symptoms of active or occult gastrointestinal bleeding, especially those at increased risk for developing ulcers, e.g., those with a history of ulcer disease or those receiving concurrent nonsteroidal anti-inflammatory drugs (NSAIDs). Clinical studies of donepezil hydrochloride in a dose of 5 mg/day to 10 mg/day have shown no increase, relative to placebo, in the incidence of either peptic ulcer disease or gastrointestinal bleeding.
5.6 Genitourinary Conditions
Although not observed in clinical trials of donepezil hydrochloride, cholinomimetics may cause bladder outflow obstruction.
5.7 Neurological Conditions: Seizures
Cholinomimetics are believed to have some potential to cause generalized convulsions. However, seizure activity also may be a manifestation of Alzheimer's disease.
5.8 Pulmonary Conditions
Because of their cholinomimetic actions, cholinesterase inhibitors should be prescribed with care to patients with a history of asthma or obstructive pulmonary disease.
•
Cholinesterase inhibitors are likely to exaggerate succinylcholine-type muscle relaxation during anesthesia (5.1).
•
Cholinesterase inhibitors may have vagotonic effects on the sinoatrial and atrioventricular nodes manifesting as bradycardia or heart block (5.2).
•
Donepezil hydrochloride can cause vomiting. Patients should be observed closely at initiation of treatment and after dose increases (5.3).
•
Patients should be monitored closely for symptoms of active or occult gastrointestinal (GI) bleeding, especially those at increased risk for developing ulcers (5.4).
•
Cholinomimetics may cause bladder outflow obstructions (5.6).
•
Cholinomimetics are believed to have some potential to cause generalized convulsions (5.7).
•
Cholinesterase inhibitors should be prescribed with care to patients with a history of asthma or obstructive pulmonary disease (5.8).
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
•
Cardiovascular Conditions [see Warnings and Precautions (5.2)]
•
Nausea and Vomiting [see Warnings and Precautions (5.3)]
•
Peptic Ulcer Disease and GI Bleeding [see Warnings and Precautions (5.4)]
•
Genitourinary Conditions [see Warnings and Precautions (5.6)]
•
Neurological Conditions: Seizures [see Warnings and Precautions (5.7)]
•
Pulmonary Conditions [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Donepezil hydrochloride has been administered to over 1,700 individuals during clinical trials worldwide. Approximately 1,200 of these patients have been treated for at least 3 months and more than 1,000 patients have been treated for at least 6 months. Controlled and uncontrolled trials in the United States included approximately 900 patients. In regards to the highest dose of 10 mg/day, this population includes 650 patients treated for 3 months, 475 patients treated for 6 months, and 116 patients treated for over 1 year. The range of patient exposure is from 1 to 1,214 days.
Mild to Moderate Alzheimer’s Disease
Adverse Reactions Leading to Discontinuation
The rates of discontinuation from controlled clinical trials of donepezil hydrochloride due to adverse reactions for the donepezil hydrochloride 5 mg/day treatment groups were comparable to those of placebo treatment groups at approximately 5%. The rate of discontinuation of patients who received 7-day escalations from 5 mg/day to 10 mg/day was higher at 13%.
The most common adverse reactions leading to discontinuation, defined as those occurring in at least 2% of patients and at twice or more the incidence seen in placebo patients, are shown in Table 1.
Table 1. Most Common Adverse Reactions Leading to Discontinuation in Patients with Mild to Moderate Alzheimer’s Disease|
Adverse Reaction |
Placebo (n=355) |
5 mg/day Donepezil Hydrochloride |
10 mg/day Donepezil Hydrochloride |
|
Nausea |
1 |
1 |
3 |
|
Diarrhea |
0 |
<1 |
3 |
|
Vomiting |
<1 |
<1 |
2 |
Most Common Adverse Reactions
The most common adverse reactions, defined as those occurring at a frequency of at least 5% in patients receiving 10 mg/day and twice the placebo rate, are largely predicted by donepezil hydrochloride’s cholinomimetic effects. These include nausea, diarrhea, insomnia, vomiting, muscle cramp, fatigue, and anorexia. These adverse reactions were often transient, resolving during continued donepezil hydrochloride treatment without the need for dose modification.
There is evidence to suggest that the frequency of these common adverse reactions may be affected by the rate of titration. An open-label study was conducted with 269 patients who received placebo in the 15- and 30-week studies. These patients were titrated to a dose of 10 mg/day over a 6-week period. The rates of common adverse reactions were lower than those seen in patients titrated to 10 mg/day over one week in the controlled clinical trials and were comparable to those seen in patients on 5 mg/day.
See Table 2 for a comparison of the most common adverse reactions following one and six week titration regimens.
Table 2. Comparison of Rates of Adverse Reactions in Mild to Moderate Patients Titrated to 10 mg/day over 1 and 6 Weeks|
No titration |
One week |
Six week titration | ||
|
Adverse Reaction |
Placebo |
5 mg/day |
10 mg/day |
10 mg/day |
|
Nausea |
6 |
5 |
19 |
6 |
|
Diarrhea |
5 |
8 |
15 |
9 |
|
Insomnia |
6 |
6 |
14 |
6 |
|
Fatigue |
3 |
4 |
8 |
3 |
|
Vomiting |
3 |
3 |
8 |
5 |
|
Muscle cramps |
2 |
6 |
8 |
3 |
|
Anorexia |
2 |
3 |
7 |
3 |
Table 3 lists adverse reactions that occurred in at least 2% of patients in pooled placebo-controlled trials who received either donepezil hydrochloride 5 mg or 10 mg and for which the rate of occurrence was greater for patients treated with donepezil hydrochloride than with placebo. In general, adverse reactions occurred more frequently in female patients and with advancing age.
Table 3. Adverse Reactions in Pooled Placebo-Controlled Clinical Trials in Mild to Moderate Alzheimer’s Disease|
Adverse Reaction |
Placebo |
Donepezil Hydrochloride |
|
Percent of Patients with any Adverse Reaction |
72 |
74 |
|
Nausea |
6 |
11 |
|
Diarrhea |
5 |
10 |
|
Headache |
9 |
10 |
|
Insomnia |
6 |
9 |
|
Pain, various locations |
8 |
9 |
|
Dizziness |
6 |
8 |
|
Accident |
6 |
7 |
|
Muscle Cramps |
2 |
6 |
|
Fatigue |
3 |
5 |
|
Vomiting |
3 |
5 |
|
Anorexia |
2 |
4 |
|
Ecchymosis |
3 |
4 |
|
Abnormal Dreams |
0 |
3 |
|
Depression |
<1 |
3 |
|
Weight Loss |
1 |
3 |
|
Arthritis |
1 |
2 |
|
Frequent Urination |
1 |
2 |
|
Somnolence |
<1 |
2 |
|
Syncope |
1 |
2 |
Severe Alzheimer’s Disease (donepezil hydrochloride 5 mg/day and 10 mg/day)
Donepezil hydrochloride has been administered to over 600 patients with severe Alzheimer’s disease during clinical trials of at least 6 months duration, including three double-blind, placebo-controlled trials, two of which had an open label extension.
Adverse Reactions Leading to Discontinuation
The rates of discontinuation from controlled clinical trials of donepezil hydrochloride due to adverse reactions for the donepezil hydrochloride patients were approximately 12% compared to 7% for placebo patients. The most common adverse reactions leading to discontinuation, defined as those occurring in at least 2% of donepezil hydrochloride patients and at twice or more the incidence seen in placebo, were anorexia (2% vs. 1% placebo), nausea (2% vs. <1% placebo), diarrhea (2% vs. 0% placebo), and urinary tract infection (2% vs. 1% placebo).
Most Common Adverse Reactions
The most common adverse reactions, defined as those occurring at a frequency of at least 5% in patients receiving donepezil hydrochloride and at twice or more the placebo rate, are largely predicted by donepezil hydrochloride’s cholinomimetic effects. These include diarrhea, anorexia, vomiting, nausea, and ecchymosis. These adverse reactions were often transient, resolving during continued donepezil hydrochloride treatment without the need for dose modification.
Table 4 lists adverse reactions that occurred in at least 2% of patients in pooled placebo-controlled trials who received donepezil hydrochloride 5 mg or 10 mg and for which the rate of occurrence was greater for patients treated with donepezil hydrochloride than with placebo.
Table 4. Adverse Reactions in Pooled Controlled Clinical Trials in Severe Alzheimer’s Disease|
Body System/Adverse Reaction |
Placebo % |
Donepezil Hydrochloride (n=501) % |
|
Percent of Patients with any Adverse Reaction |
73 |
81 |
|
Accident |
12 |
13 |
|
Infection |
9 |
11 |
|
Diarrhea |
3 |
4 |
|
Anorexia |
2 |
3 |
|
Vomiting |
2 |
3 |
|
Nausea |
1 |
2 |
|
Insomnia |
<1 |
2 |
|
Ecchymosis |
2 |
3 |
|
Headache |
1 |
2 |
|
Pain |
1 |
2 |
|
Back Pain |
4 |
10 |
|
Eczema |
4 |
8 |
|
Hallucinations |
4 |
8 |
|
Hostility |
2 |
6 |
|
Increase in Creatine Phosphokinase |
2 |
5 |
|
Nervousness |
1 |
3 |
|
Fever |
1 |
2 |
|
Chest Pain |
<1 |
2 |
|
Confusion |
4 |
5 |
|
Dehydration |
2 |
3 |
|
Depression |
2 |
3 |
|
Dizziness |
1 |
3 |
|
Emotional Lability |
1 |
2 |
|
Hemorrhage |
1 |
2 |
|
Hyperlipemia |
1 |
2 |
|
Personality Disorder |
1 |
2 |
|
Somnolence |
1 |
2 |
|
Personality Disorder |
1 |
2 |
|
Syncope |
2 |
3 |
|
Urinary Incontinence |
1 |
2 |
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of donepezil hydrochloride. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Abdominal pain, agitation, aggression, cholecystitis, confusion, convulsions, hallucinations, heart block (all types), hemolytic anemia, hepatitis, hyponatremia, neuroleptic malignant syndrome, pancreatitis, rash, rhabdomyolysis, QTc prolongation, and torsade de pointes.
Most common adverse reactions in clinical studies of donepezil hydrochloride are nausea, diarrhea, insomnia, vomiting, muscle cramps, fatigue, and anorexia (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Solco Healthcare US, LLC at 1-866-257-2597 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DESCRIPTION SECTION
11 DESCRIPTION
Donepezil hydrochloride is a reversible inhibitor of the enzyme acetylcholinesterase, known chemically as (±)-2, 3-dihydro-5, 6-dimethoxy-2-[[1-(phenylmethyl)-4-piperidinyl]methyl]-1H-inden-1-one hydrochloride. Donepezil hydrochloride is commonly referred to in the pharmacological literature as E2020. It has an empirical formula of C24H29NO3HCl and a molecular weight of 415.96. Donepezil hydrochloride is a white crystalline powder and is freely soluble in chloroform, soluble in water and in glacial acetic acid, slightly soluble in ethanol and in acetonitrile, and practically insoluble in ethyl acetate and in n-hexane.
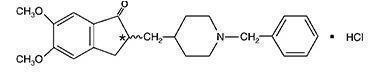
Donepezil Hydrochloride Tablets, USP, are available for oral administration in film-coated tablets containing 5 mg or 10 mg of donepezil hydrochloride, USP.
Inactive ingredients in 5 mg and 10 mg tablets are corn starch, croscarmellose sodium, hydroxypropyl cellulose, lactose monohydrate, magnesium Stearate, microcrystalline cellulose. Additionally, the 5 mg tablets coating film contains FD&C Blue #2/INDIGO CARMINE ALUMINUM LAKE, hypromellose, polyethylene glycol, and titanium dioxide. The 10 mg tablets coating film contains hypromellose, iron oxide red, iron oxide yellow, polyethylene glycol, and titanium dioxide.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 Donepezil Hydrochloride Tablets
Supplied as film-coated, round tablets containing 5 mg, or 10 mg of donepezil hydrochloride, USP.
The 5 mg tablets are blue, round, film-coated tablets, debossed with ‘HH205' on one side.
•
Bottles of 30 (NDC# 43547-878-03)
•
Bottles of 90 (NDC# 43547-878-09)
•
Bottles of 1,000 (NDC# 43547-878-11)
The 10 mg tablets are light yellow, round, film-coated tablets, debossed with ‘HH210' on one side.
•
Bottles of 30 (NDC# 43547-879-03)
•
Bottles of 90 (NDC# 43547-879-09)
•
Bottles of 1,000 (NDC# 43547-879-11)
Storage
Store at controlled room temperature, 15°C to 30°C (59°F to 86°F).
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Instruct patients and caregivers to take donepezil hydrochloride only once per day, as prescribed.
Instruct patients and caregivers that donepezil hydrochloride can be taken with or without food.
Advise patients and caregivers that donepezil hydrochloride may cause nausea, diarrhea, insomnia, vomiting, muscle cramps, fatigue, and decreased appetite.
Advise patients to notify their healthcare provider if they are pregnant or plan to become pregnant.
Manufactured by:
Prinston Laboratories
Charlotte, NC 28206, USA
Distributed by:
Solco Healthcare US, LLC
Somerset, NJ 08873, USA
Revised: 06/2025
9040684-01
DONEPEZIL HYDROCHLORIDE PATIENT PACKAGE INSERT
Donepezil (doe NEP e zil) Hydrochloride Tablets, USP
•
Tablets: 5 mg and 10 mg
Read this Patient Information that comes with donepezil hydrochloride before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about Alzheimer’s disease or treatment for it. If you have questions, ask the doctor or pharmacist.
What is donepezil hydrochloride?
Donepezil hydrochloride comes as donepezil hydrochloride film-coated tablets in dosage strengths of 5 mg and 10 mg.
Donepezil hydrochloride is a prescription medicine to treat mild, moderate and severe Alzheimer’s disease. Donepezil hydrochloride can help with mental function and with doing daily tasks. Donepezil hydrochloride does not work the same in all people. Some people may:
•
Seem much better
•
Get better in small ways or stay the same
•
Get worse over time but slower than expected
•
Not change and then get worse as expected
Donepezil hydrochloride does not cure Alzheimer's disease. All patients with Alzheimer's disease get worse over time, even if they take donepezil hydrochloride.
Donepezil hydrochloride has not been approved as a treatment for any medical condition in children.
Who should not take donepezil hydrochloride?
Do not take donepezil hydrochloride if you are allergic to any of the ingredients in donepezil hydrochloride tablets or to medicines that contain piperidines. Ask your doctor if you are not sure. See the end of this leaflet for a list of ingredients in donepezil hydrochloride tablets.
What should I tell my doctor before taking donepezil hydrochloride?
Tell the doctor about all of your present or past health problems and conditions. Include:
•
Any heart problems including problems with irregular, slow, or fast heartbeats
•
Asthma or lung problems
•
A seizure
•
Stomach ulcers
•
Difficulty passing urine
•
Liver or kidney problems
•
Trouble swallowing tablets
•
Present pregnancy or plans to become pregnant. It is not known if donepezil hydrochloride can harm an unborn baby.
•
Present breast-feeding. It is not known if donepezil hydrochloride passes into breast milk. Talk to your doctor about the best way to feed your baby if you take donepezil hydrochloride.
Tell the doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal products. Donepezil hydrochloride and other medicines may affect each other.
Be particularly sure to tell the doctor if you take aspirin or medicines called nonsteroidal anti-inflammatory drugs (NSAIDs). There are many NSAID medicines, both prescription and non-prescription. Ask the doctor or pharmacist if you are not sure if any of your medicines are NSAIDs. Taking NSAIDs and donepezil hydrochloride together may make you more likely to get stomach ulcers.
Donepezil hydrochloride taken with certain medicines used for anesthesia may cause side effects. Tell the responsible doctor or dentist that you take donepezil hydrochloride before you have:
•
surgery
•
medical procedures
•
dental surgery or procedures
Know the medicines that you take. Keep a list of all your medicines. Show it to your doctor or pharmacist before you start a new medicine.
How should you take donepezil hydrochloride?
•
Take donepezil hydrochloride exactly as prescribed by the doctor. Do not stop donepezil hydrochloride or change the dose yourself. Talk with your doctor first.
•
Take donepezil hydrochloride one time each day. Donepezil hydrochloride can be taken with or without food.
•
If you miss giving the patient a dose of donepezil hydrochloride, just wait. Take only the next dose at the usual time. Do not take 2 doses at the same time.
•
If donepezil hydrochloride is missed for 7 days or more, talk with your doctor before starting again.
•
If you take too much donepezil hydrochloride at one time, call your doctor or poison control center, or go to the emergency room right away.
What are the possible side effects of donepezil hydrochloride?
Donepezil hydrochloride may cause the following serious side effects:
•
**slow heartbeat and fainting.** This happens more often in people with heart problems. Call your doctor right away if you feel faint or lightheaded while taking donepezil hydrochloride.
•
**more stomach acid.** This raises the chance of ulcers and bleeding. The risk is higher for people who have ulcers, or take aspirin or other NSAIDs.
•
worsening of lung problems in people with asthma or other lung disease.
•
seizures.
•
difficulty passing urine.
Call your doctor right away if you have:
•
fainting.
•
heartburn or stomach pain that is new or won't go away.
•
nausea or vomiting, blood in the vomit, dark vomit that looks like coffee grounds.
•
bowel movements or stools that look like black tar.
•
new or worse asthma or breathing problems.
•
seizures.
•
difficulty passing urine.
The most common side effects of donepezil hydrochloride are:
•
nausea
•
diarrhea
•
not sleeping well
•
vomiting
•
muscle cramps
•
feeling tired
•
not wanting to eat
These side effects may get better after you take donepezil hydrochloride for a while. This is not a complete list of side effects with donepezil hydrochloride. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to Solco Healthcare U. S., LLC at 1-866-257-2597 or FDA at 1-800-FDA-1088.
How should donepezil hydrochloride be stored?
Store donepezil hydrochloride at room temperature between 59° to 86°F (15° to 30°C).
Keep donepezil hydrochloride and all medicines out of the reach of children.
General information about donepezil hydrochloride
Medicines are sometimes prescribed for conditions that are not mentioned in this Patient Information Leaflet. Do not use donepezil hydrochloride for a condition for which it was not prescribed. Do not give donepezil hydrochloride to other people, even if they have the same symptoms or condition. It may harm them.
This leaflet summarizes the most important information about donepezil hydrochloride. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about donepezil hydrochloride that is written for health professionals.
What are the ingredients in Donepezil Hydrochloride Tablets, USP?
Active ingredient: donepezil hydrochloride
Inactive ingredients:
**Donepezil Hydrochloride 5 mg and 10 mg film-coated tablets:**corn starch, croscarmellose sodium, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose. The film of 5 mg tablets coating contains FD&C BLUE #2/INDIGO CARMINE ALUMINUM LAKE, hypromellose, polyethylene glycol, and titanium dioxide. The film of 10 mg tablets coating contains hypromellose, iron oxide red, iron oxide yellow, polyethylene glycol, and titanium dioxide.
Rx Only
Manufactured by:
Prinston Laboratories
Charlotte, NC 28206, USA
Distributed by:
Solco Healthcare US, LLC
Somerset, NJ 08873, USA
Revised: 06/2025
Rx Only
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Mild to Moderate Alzheimer's Disease
The effectiveness of donepezil hydrochloride as a treatment for mild to moderate Alzheimer's disease is demonstrated by the results of two randomized, double-blind, placebo-controlled clinical investigations in patients with Alzheimer's disease (diagnosed by NINCDS and DSM III-R criteria, Mini-Mental State Examination ≥ 10 and ≤ 26 and Clinical Dementia Rating of 1 or 2). The mean age of patients participating in donepezil hydrochloride trials was 73 years with a range of 50 to 94. Approximately 62% of patients were women and 38% were men. The racial distribution was white 95%, black 3%, and other races 2%.
The higher dose of 10 mg did not provide a statistically significantly greater clinical benefit than 5 mg. There is a suggestion, however, based upon order of group mean scores and dose trend analyses of data from these clinical trials, that a daily dose of 10 mg of donepezil hydrochloride might provide additional benefit for some patients. Accordingly, whether or not to employ a dose of 10 mg is a matter of prescriber and patient preference.
Study Outcome Measures
In each study, the effectiveness of treatment with donepezil hydrochloride was evaluated using a dual outcome assessment strategy.
The ability of donepezil hydrochloride to improve cognitive performance was assessed with the cognitive subscale of the Alzheimer's Disease Assessment Scale (ADAS-cog), a multi-item instrument that has been extensively validated in longitudinal cohorts of Alzheimer's disease patients. The ADAS-cog examines selected aspects of cognitive performance including elements of memory, orientation, attention, reasoning, language, and praxis. The ADAS-cog scoring range is from 0 to 70, with higher scores indicating greater cognitive impairment. Elderly normal adults may score as low as 0 or 1, but it is not unusual for non-demented adults to score slightly higher.
The patients recruited as participants in each study had mean scores on the ADAS-cog of approximately 26 points, with a range from 4 to 61. Experience based on longitudinal studies of ambulatory patients with mild to moderate Alzheimer's disease suggest that scores on the ADAS-cog increase (worsen) by 6-12 points per year. However, smaller changes may be seen in patients with very mild or very advanced disease since the ADAS-cog is not uniformly sensitive to change over the course of the disease. The annualized rate of decline in the placebo patients participating in donepezil hydrochloride trials was approximately 2 to 4 points per year.
The ability of donepezil hydrochloride to produce an overall clinical effect was assessed using a Clinician's Interview-Based Impression of Change that required the use of caregiver information, the CIBIC-plus. The CIBIC-plus is not a single instrument and is not a standardized instrument like the ADAS- cog. Clinical trials for investigational drugs have used a variety of CIBIC formats, each different in terms of depth and structure.
As such, results from a CIBIC-plus reflect clinical experience from the trial or trials in which it was used and cannot be compared directly with the results of CIBIC-plus evaluations from other clinical trials. The CIBIC-plus used in donepezil hydrochloride trials was a semi-structured instrument that was intended to examine four major areas of patient function: General, Cognitive, Behavioral, and Activities of Daily Living. It represents the assessment of a skilled clinician based upon his/her observations at an interview with the patient, in combination with information supplied by a caregiver familiar with the behavior of the patient over the interval rated. The CIBIC-plus is scored as a seven-point categorical rating, ranging from a score of 1, indicating “markedly improved,” to a score of 4, indicating “no change” to a score of 7, indicating “markedly worse.” The CIBIC-plus has not been systematically compared directly to assessments not using information from caregivers (CIBIC) or other global methods.
Thirty-Week Study
In a study of 30 weeks duration, 473 patients were randomized to receive single daily doses of placebo, 5 mg/day or 10 mg/day of donepezil hydrochloride. The 30-week study was divided into a 24-week double-blind active treatment phase followed by a 6-week single-blind placebo washout period. The study was designed to compare 5 mg/day or 10 mg/day fixed doses of donepezil hydrochloride to placebo. However, to reduce the likelihood of cholinergic effects, the 10 mg/day treatment was started following an initial 7-day treatment with 5 mg/day doses.
Effects on the ADAS-cog
Figure 1 illustrates the time course for the change from baseline in ADAS-cog scores for all three dose groups over the 30 weeks of the study. After 24 weeks of treatment, the mean differences in the ADAS-cog change scores for donepezil hydrochloride treated patients compared to the patients on placebo were 2.8 and 3.1 points for the 5 mg/day and 10 mg/day treatments, respectively. These differences were statistically significant. While the treatment effect size may appear to be slightly greater for the 10 mg/day treatment, there was no statistically significant difference between the two active treatments.
Following 6 weeks of placebo washout, scores on the ADAS-cog for both the donepezil hydrochloride treatment groups were indistinguishable from those patients who had received only placebo for 30 weeks. This suggests that the beneficial effects of donepezil hydrochloride abate over 6 weeks following discontinuation of treatment and do not represent a change in the underlying disease. There was no evidence of a rebound effect 6 weeks after abrupt discontinuation of therapy.
Figure 1. Time-course of the Change from Baseline in ADAS-cog Score for Patients Completing 24 Weeks of Treatment
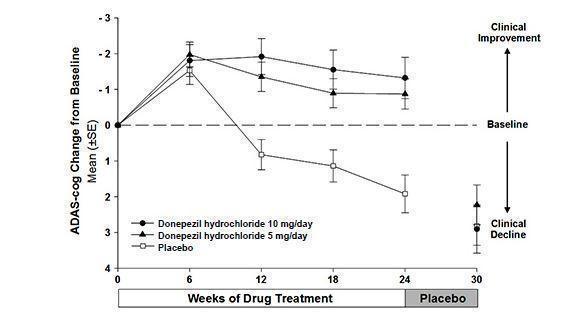
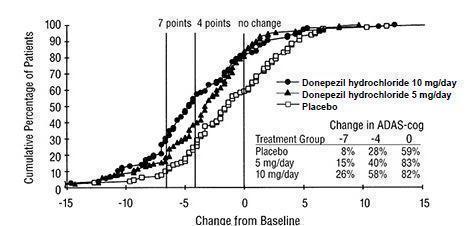
Figure 2 illustrates the cumulative percentages of patients from each of the three treatment groups who had attained the measure of improvement in ADAS-cog score shown on the X axis. Three change scores (7-point and 4-point reductions from baseline or no change in score) have been identified for illustrative purposes, and the percent of patients in each group achieving that result is shown in the inset table.
The curves demonstrate that both patients assigned to placebo and donepezil hydrochloride have a wide range of responses, but that the active treatment groups are more likely to show greater improvements. A curve for an effective treatment would be shifted to the left of the curve for placebo, while an ineffective or deleterious treatment would be superimposed upon or shifted to the right of the curve for placebo.
Figure 2. Cumulative Percentage of Patients Completing 24 Weeks of Double- blind Treatment with Specified Changes from Baseline ADAS-cog Scores. The Percentages of Randomized Patients who Completed the Study were: Placebo 80%, 5 mg/day 85%, and 10 mg/day 68%.
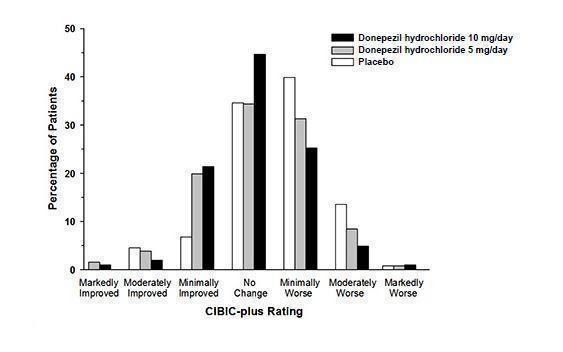
Effects on the CIBIC-plus
Figure 3 is a histogram of the frequency distribution of CIBIC-plus scores attained by patients assigned to each of the three treatment groups who completed 24 weeks of treatment. The mean drug-placebo differences for these groups of patients were 0.35 points and 0.39 points for 5 mg/day and 10 mg/day of donepezil hydrochloride, respectively. These differences were statistically significant. There was no statistically significant difference between the two active treatments.
Figure 3. Frequency Distribution of CIBIC-plus Scores at Week 24.
Fifteen-Week Study
In a study of 15 weeks duration, patients were randomized to receive single daily doses of placebo or either 5 mg/day or 10 mg/day of donepezil hydrochloride for 12 weeks, followed by a 3-week placebo washout period. As in the 30-week study, to avoid acute cholinergic effects, the 10 mg/day treatment followed an initial 7-day treatment with 5 mg/day doses.
Effects on the ADAS-cog
Figure 4 illustrates the time course of the change from baseline in ADAS-cog scores for all three dose groups over the 15 weeks of the study. After 12 weeks of treatment, the differences in mean ADAS-cog change scores for the donepezil hydrochloride treated patients compared to the patients on placebo were 2.7 and 3.0 points each, for the 5 and 10 mg/day donepezil hydrochloride treatment groups, respectively. These differences were statistically significant. The effect size for the 10 mg/day group may appear to be slightly larger than that for 5 mg/day. However, the differences between active treatments were not statistically significant.
Figure 4. Time-course of the Change from Baseline in ADAS-cog Score for Patients Completing the 15-week Study.
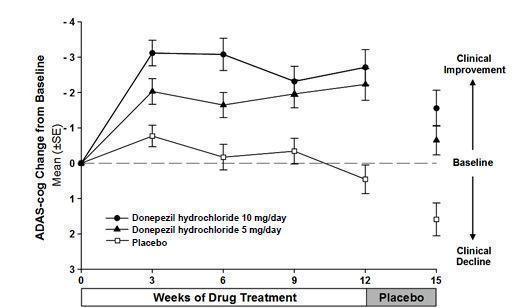
Following 3 weeks of placebo washout, scores on the ADAS-cog for both the donepezil hydrochloride treatment groups increased, indicating that discontinuation of donepezil hydrochloride resulted in a loss of its treatment effect. The duration of this placebo washout period was not sufficient to characterize the rate of loss of the treatment effect, but the 30-week study (see above) demonstrated that treatment effects associated with the use of donepezil hydrochloride abate within 6 weeks of treatment discontinuation.
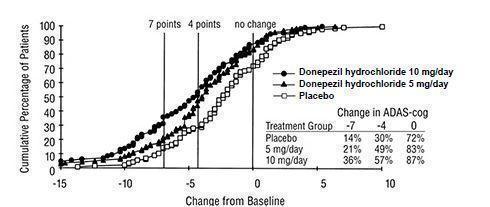
Figure 5 illustrates the cumulative percentages of patients from each of the three treatment groups who attained the measure of improvement in ADAS-cog score shown on the X axis. The same three change scores (7-point and 4-point reductions from baseline or no change in score) as selected for the 30-week study have been used for this illustration. The percentages of patients achieving those results are shown in the inset table.
As observed in the 30-week study, the curves demonstrate that patients assigned to either placebo or to donepezil hydrochloride have a wide range of responses, but that the donepezil hydrochloride treated patients are more likely to show greater improvements in cognitive performance.
Figure 5. Cumulative Percentage of Patients with Specified Changes from Baseline ADAS-cog Scores. The Percentages of Randomized Patients Within Each Treatment Group Who Completed the Study Were: Placebo 93%, 5 mg/day 90%, and 10 mg/day 82%.
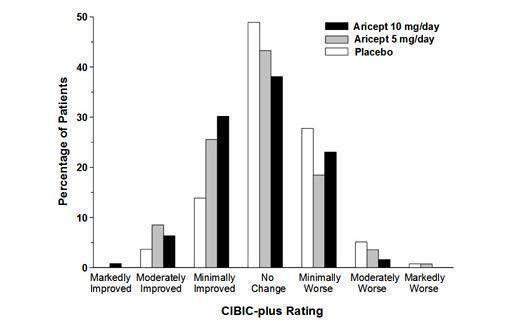
Effects on the CIBIC-plus
Figure 6 is a histogram of the frequency distribution of CIBIC-plus scores attained by patients assigned to each of the three treatment groups who completed 12 weeks of treatment. The differences in mean scores for donepezil hydrochloride treated patients compared to the patients on placebo at Week 12 were 0.36 and 0.38 points for the 5 mg/day and 10 mg/day treatment groups, respectively. These differences were statistically significant.
Figure 6. Frequency Distribution of CIBIC-plus Scores at Week 12.
In both studies, patient age, sex and race were not found to predict the clinical outcome of donepezil hydrochloride treatment.
14.2 Moderate to Severe Alzheimer’s Disease
The effectiveness of donepezil hydrochloride in the treatment of patients with moderate to severe Alzheimer’s disease was established in studies employing doses of 10 mg/day.
Swedish 6 Month Study (10 mg/day)
The effectiveness of donepezil hydrochloride as a treatment for severe Alzheimer's disease is demonstrated by the results of a randomized, double- blind, placebo-controlled clinical study conducted in Sweden (6 month study) in patients with probable or possible Alzheimer's disease diagnosed by NINCDS- ADRDA and DSM-IV criteria, MMSE: range of 1-10. Two hundred and forty eight (248) patients with severe Alzheimer's disease were randomized to donepezil hydrochloride or placebo. For patients randomized to donepezil hydrochloride, treatment was initiated at 5 mg once daily for 28 days and then increased to 10 mg once daily. At the end of the 6 month treatment period, 90.5% of the donepezil hydrochloride treated patients were receiving the 10 mg/day dose. The mean age of patients was 84.9 years, with a range of 59 to 99. Approximately 77 % of patients were women, and 23 % were men. Almost all patients were Caucasian. Probable Alzheimer’s disease was diagnosed in the majority of the patients (83.6% of donepezil hydrochloride treated patients and 84.2% of placebo treated patients).
Study Outcome Measures
The effectiveness of treatment with donepezil hydrochloride was determined using a dual outcome assessment strategy that evaluated cognitive function using an instrument designed for more impaired patients and overall function through caregiver-rated assessment. This study showed that patients on donepezil hydrochloride experienced significant improvement on both measures compared to placebo.
The ability of donepezil hydrochloride to improve cognitive performance was assessed with the Severe Impairment Battery (SIB). The SIB, a multi-item instrument, has been validated for the evaluation of cognitive function in patients with moderate to severe dementia. The SIB evaluates selective aspects of cognitive performance, including elements of memory, language, orientation, attention, praxis, visuospatial ability, construction, and social interaction. The SIB scoring range is from 0 to 100, with lower scores indicating greater cognitive impairment.
Daily function was assessed using the Modified Alzheimer's Disease Cooperative Study Activities of Daily Living Inventory for Severe Alzheimer's Disease (ADCS-ADL-severe). The ADCS-ADL-severe is derived from the Alzheimer's Disease Cooperative Study Activities of Daily Living Inventory, which is a comprehensive battery of ADL questions used to measure the functional capabilities of patients. Each ADL item is rated from the highest level of independent performance to complete loss. The ADCS-ADL-severe is a subset of 19 items, including ratings of the patient's ability to eat, dress, bathe, use the telephone, get around (or travel), and perform other activities of daily living; it has been validated for the assessment of patients with moderate to severe dementia. The ADCS-ADL-severe has a scoring range of 0 to 54, with the lower scores indicating greater functional impairment. The investigator performs the inventory by interviewing a caregiver, in this study a nurse staff member, familiar with the functioning of the patient.
Effects on the SIB
Figure 7 shows the time course for the change from baseline in SIB score for the two treatment groups over the 6 months of the study. At 6 months of treatment, the mean difference in the SIB change scores for donepezil hydrochloride treated patients compared to patients on placebo was 5.9 points. Donepezil hydrochloride treatment was statistically significantly superior to placebo.
Figure 7. Time Course of the Change from Baseline in SIB Score for Patients Completing 6 Months of Treatment.
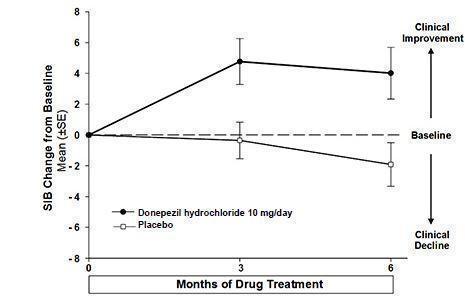
Figure 8 illustrates the cumulative percentages of patients from each of the two treatment groups who attained the measure of improvement in SIB score shown on the X-axis. While patients assigned both to donepezil hydrochloride and to placebo have a wide range of responses, the curves show that the donepezil hydrochloride group is more likely to show a greater improvement in cognitive performance.
Figure 8. Cumulative Percentage of Patients Completing 6 Months of Double- blind Treatment with Particular Changes from Baseline in SIB Scores.
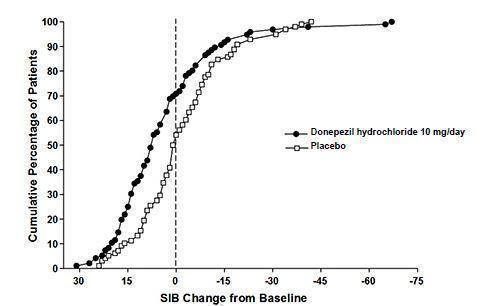
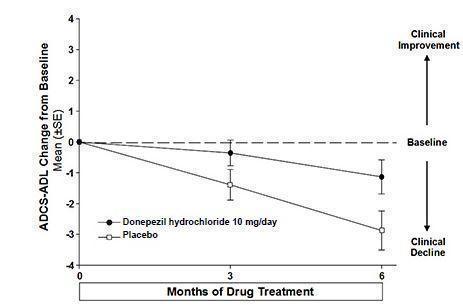
Figure 9. Time Course of the Change from Baseline in ADCS-ADL-Severe Score for Patients Completing 6 Months of Treatment.
Effects on the ADCS-ADL-severe
Figure 9 illustrates the time course for the change from baseline in ADCS-ADL- severe scores for patients in the two treatment groups over the 6 months of the study. After 6 months of treatment, the mean difference in the ADCS-ADL- severe change scores for donepezil hydrochloride treated patients compared to patients on placebo was 1.8 points. Donepezil hydrochloride treatment was statistically significantly superior to placebo.
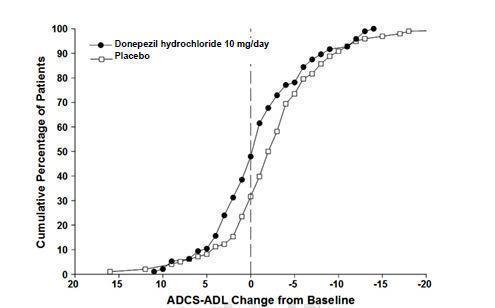
Figure 10 shows the cumulative percentages of patients from each treatment group with specified changes from baseline ADCS-ADL-severe scores. While both patients assigned to donepezil hydrochloride and placebo have a wide range of responses, the curves demonstrate that the donepezil hydrochloride group is more likely to show a smaller decline or an improvement.
Figure 10. Cumulative Percentage of Patients Completing 6 Months of Double- blind Treatment with Particular Changes from Baseline in ADCS-ADL-Severe Scores.
Japanese 24-Week Study (10 mg/day)
In a study of 24 weeks duration conducted in Japan, 325 patients with severe Alzheimer's disease were randomized to doses of 5 mg/day or 10 mg/day of donepezil, administered once daily, or placebo. Patients randomized to treatment with donepezil were to achieve their assigned doses by titration, beginning at 3 mg/day, and extending over a maximum of 6 weeks. Two hundred and forty eight (248) patients completed the study, with similar proportions of patients completing the study in each treatment group. The primary efficacy measures for this study were the SIB and CIBIC-plus.
At 24 weeks of treatment, statistically significant treatment differences were observed between the 10 mg/day dose of donepezil and placebo on both the SIB and CIBIC-plus. The 5 mg/day dose of donepezil showed a statistically significant superiority to placebo on the SIB, but not on the CIBIC-plus.
