Pulmozyme
These highlights do not include all the information needed to use PULMOZYME safely and effectively. See full prescribing information for PULMOZYME. PULMOZYME (dornase alfa) inhalation solution, for inhalation use Initial U.S. Approval: 1993
d8c78a7e-ff99-48f3-8952-643ec2ea0f86
HUMAN PRESCRIPTION DRUG LABEL
Feb 16, 2024
Genentech, Inc.
DUNS: 080129000
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
dornase alfa
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
Drug Labeling Information
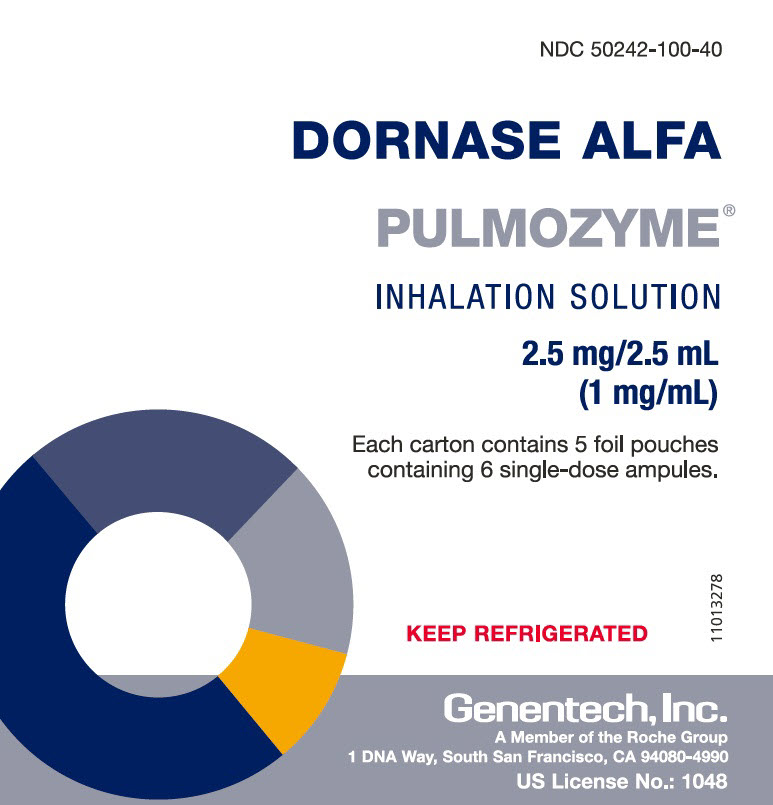
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 2.5 mg/2.5 mL Ampule Pouch Carton
NDC 50242-100-40
DORNASE ALFA
PULMOZYME®
INHALATION SOLUTION
2.5 mg/2.5 mL
(1 mg/mL)
Each carton contains 5 foil pouches
containing 6 single-dose ampules.
KEEP REFRIGERATED
11013278
Genentech, Inc.
A Member of the Roche Group
1 DNA Way, South San Francisco, CA 94080-4990
US License No.: 1048
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
PULMOZYME® is indicated, in conjunction with standard therapies, for the management of pediatric and adult patients with cystic fibrosis (CF) to improve pulmonary function.
In CF patients with an FVC ≥ 40% of predicted, daily administration of PULMOZYME has also been shown to reduce the risk of respiratory tract infections requiring parenteral antibiotics.
PULMOZYME is a recombinant DNase enzyme indicated in conjunction with standard therapies for the management of cystic fibrosis (CF) patients to improve pulmonary function. (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
PULMOZYME is contraindicated in patients with known hypersensitivity to dornase alfa, Chinese Hamster Ovary cell products, or any component of the product.
PULMOZYME is contraindicated in patients with known hypersensitivity to dornase alfa, Chinese Hamster Ovary cell products, or any component of the product. (4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
None.
None. (5)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to PULMOZYME in 902 patients, with exposures ranging from 2 weeks of daily administration up to once or twice daily administration for six months. PULMOZYME was studied in both placebo- controlled (n=804) and uncontrolled trials (n=98). The population of patients in placebo-controlled trials was with FVC ≥ 40% of predicted (n=643) or with more advanced pulmonary disease, FVC < 40% of predicted (n=161). The population in the uncontrolled trial included 98 pediatric patients with CF ranging from 3 months to 10 years of age. More than half of the patients received PULMOZYME 2.5 mg by inhalation once a day (n=581), while the rest of patients (n=321) received PULMOZYME 2.5 mg by inhalation twice a day.
Placebo-Controlled Trials
Trial 1: Trial 1 was a randomized, placebo-controlled clinical trial in patients with FVC ≥ 40% of predicted. In this trial, over 600 patients received PULMOZYME once or twice daily for six months. The most common adverse reaction (risk difference ≥5%) was voice alteration. The proportion of most adverse events was similar for patients on PULMOZYME and on placebo, probably reflecting the sequelae of the underlying lung disease. In most cases reactions that were increased were mild, transient in nature, and did not require alterations in dosing. Few patients experienced adverse reactions resulting in permanent discontinuation from PULMOZYME, and the proportion of discontinuations were similar for placebo (2%) and PULMOZYME (3%). Adverse reactions occurring in a higher proportion (greater than 3%) of PULMOZYME treated patients than in placebo-treated patients are listed in Table 2.
Trial 2: Trial 2 was a randomized, placebo-controlled trial in patients with more advanced pulmonary disease (FVC < 40% of predicted) who were treated for 12 weeks. In this trial, the safety profile of PULMOZYME was similar to that reported in patients with less advanced pulmonary disease (FVC ≥ 40% of predicted). Adverse reactions that were reported in this trial with a higher proportion (greater than 3%) in the PULMOZYME treated patients are listed in Table 2.
Table 2. Adverse Reactions Increased 3% or More in PULMOZYME Treated Patients Over Placebo in CF Clinical Trials|
Adverse Reactions |
Trial 1 |
Trial 2 | |||
|---|---|---|---|---|---|
|
Placebo |
Pulmozyme QD |
Pulmozyme BID |
Placebo |
Pulmozyme QD | |
| |||||
|
Voice alteration |
7% |
12% |
16% |
6% |
18% |
|
Pharyngitis |
33% |
36% |
40% |
28% |
32% |
|
Rash |
7% |
10% |
12% |
1% |
3% |
|
Laryngitis |
1% |
3% |
4% |
1% |
3% |
|
Chest Pain |
16% |
18% |
21% |
23% |
25% |
|
Conjunctivitis |
2% |
4% |
5% |
0% |
1% |
|
Rhinitis |
Differences were less than 3% |
24% |
30% | ||
|
FVC decrease of ≥ 10% of predicted* |
17% |
22% | |||
|
Fever |
28% |
32% | |||
|
Dyspepsia |
0% |
3% | |||
|
Dyspnea (when reported as serious) |
Differences were less than 3% |
12%† |
17%† |
Mortality rates observed in controlled trials were similar for the placebo and PULMOZYME treated patients. Causes of death were consistent with progression of cystic fibrosis and included apnea, cardiac arrest, cardiopulmonary arrest, cor pulmonale, heart failure, massive hemoptysis, pneumonia, pneumothorax, and respiratory failure.
Uncontrolled Trial
Trial 3: The safety of PULMOZYME, 2.5 mg by inhalation, was studied with 2 weeks of daily administration in 98 pediatric patients with cystic fibrosis 3 months to 10 years of age (65 aged 3 months to < 5 years, 33 aged 5 to ≤ 10 years). The PARI BABY™ reusable nebulizer (which uses a facemask instead of a mouthpiece) was utilized in patients unable to demonstrate the ability to inhale or exhale orally throughout the entire treatment period (54/65, 83% of the younger and 2/33, 6% of the older patients). Overall, the nature of adverse reactions was similar to that seen in the placebo-controlled trials. The number of patients reporting cough was higher in the younger age group as compared to the older age group (29/65, 45% compared to 10/33, 30%) as was the number reporting moderate to severe cough (24/65, 37% as compared to 6/33, 18%). The number of patients reporting rhinitis was higher in the younger age group as compared to the older age group (23/65, 35% compared to 9/33, 27%) as was the number reporting rash (4/65, 6% as compared to 0/33).
Allergic Reactions
There have been no reports of anaphylaxis attributed to the administration of PULMOZYME. Urticaria, mild to moderate, and mild skin rash have been observed and have been transient. Within all of the studies, a small percentage (average of 2-4%) of patients treated with PULMOZYME developed serum antibodies to PULMOZYME. None of these patients developed anaphylaxis, and the clinical significance of serum antibodies to PULMOZYME is unknown.
The most common adverse reactions (occurring in ≥3% of patients treated with PULMOZYME over placebo) seen in clinical trials in CF patients were: voice alteration, pharyngitis, rash, laryngitis, chest pain, conjunctivitis, rhinitis, decrease in FVC of ≥10%, fever, and dyspnea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Genentech at 1-888-835-2555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
Available data indicate there are no clinically important drug-drug interactions with PULMOZYME.
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
|
Dosage and Administration. (2.1, 2.2) |
02/2024 |
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage, in most cystic fibrosis patients, is 2.5 mg (one single-dose ampule) inhaled once daily using a recommended jet nebulizer connected to an air compressor system or via a vibrating mesh nebulizer [see Dosage and Administration (2.2)].
Some patients may benefit from twice daily administration [see Clinical Studies (14)].
2.2 Administration Instructions
Nebulizer Information
- Administer PULMOZYME via a jet nebulizer connected to an air compressor with an adequate air flow and equipped with a mouthpiece or suitable face mask, or via a vibrating mesh nebulizer. Refer to Table 1 for the recommended Jet Nebulizers or Vibrating Mesh Nebulizers for use with PULMOZYME. No data are currently available to support the administration of PULMOZYME with other nebulizer systems.
- The eRapid Nebulizer System should only be used by adults and pediatric patients who can use a mouthpiece, and not by younger patients who need a mask to inhale PULMOZYME.
- Use the selected nebulizer in accordance with the manufacturer's instruction manual.
- Refer to the manufacturer's instruction manual on the use, maintenance, and replacement of the equipment, including cleaning and disinfection procedures for the selected nebulizer.
- For additional information, refer to the selected nebulizer manufacturer's instruction manual.
|
Jet Nebulizer* |
Compressor |
|---|---|
| |
|
Hudson T Up-draft II® |
Pulmo-Aide® or legally marketed compressor of identical pressure and flow rate (maximum 30 psi, 12 LPM). |
|
Marquest Acorn II® | |
|
PARI LC® Plus |
PARI PRONEB® or legally marketed compressor of identical pressure and flow rate (maximum 24 psi, 9 LPM). |
|
PARI BABY™† | |
|
Durable Sidestream® |
MOBILAIRE™, Porta-NEB® or legally marketed compressor of identical pressure and flow rate (maximum 45 psi, 7 LPM). |
|
Vibrating Mesh Nebulizers***** | |
|
eRapid® Nebulizer System‡ | |
|
Innospire Go | |
|
Pulmogine Vibrating Mesh Nebulizer | |
|
AireHealth Nebulizer™ | |
|
Intelligent Mesh Nebulizer |
PULMOZYME Information
- Each PULMOZYME ampule should be squeezed prior to use in order to check for leaks. Discard ampules if the solution is cloudy or discolored. Once opened, the entire contents of the ampule must be used or discarded.
- Do not dilute or mix PULMOZYME with other drugs in the nebulizer. Mixing of PULMOZYME with other drugs could lead to adverse physicochemical and/or functional changes in PULMOZYME or the admixed compound.
- The recommended dosage is 2.5 mg (one single-dose ampule) inhaled once daily using a recommended nebulizer. (2.1)
- Some patients may benefit from twice daily administration. (2.1)
- See full prescribing information for the recommended nebulizers for use with PULMOZYME. (2.2)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Inhalation solution: 2.5 mg/2.5 mL (1 mg/mL) clear, colorless solution in single-dose ampules.
Inhalation solution: 2.5 mg/2.5 mL (1 mg/mL) clear, colorless solution in single-dose ampules (3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies with PULMOZYME in pregnant women. However, animal reproduction studies have been conducted with dornase alfa. In these studies, no evidence of fetal harm was observed in rats and rabbits at doses of dornase alfa up to approximately 600 times the maximum recommended human dose (MRHD).
The background risk of major birth defects and miscarriage for the cystic fibrosis population is unknown. However, the background risk in the U.S. general population of major birth defects is 2-4% and of miscarriage is 15-20% of clinically recognized pregnancies.
Data
Animal Data
Reproductive studies have been performed in rats and rabbits at intravenous doses of dornase alfa up to 10 mg/kg/day (approximately 600 times the MRHD in adults). In a combined embryo-fetal development and pre- and post-natal development study, no evidence of maternal toxicity, embryotoxicity, or teratogenicity was observed when dornase alfa was administered to dams throughout organogenesis (Gestation days 6 to 17). Dornase alfa did not elicit adverse effects on fetal or neonatal growth when administered to dams throughout most of gestation and delivery (Gestation days 6 to 25) and nursing (Post-partum days 6 to 21).
A pharmacokinetic study in Cynomolgus monkeys found no detectable levels of dornase alfa in fetal blood or amniotic fluid on gestation day 150 (end of gestation) from mothers that were administered an intravenous bolus dose (0.1 mg/kg) followed by an intravenous infusion dose (0.080 mg/kg) over a 6-hour period during pregnancy.
8.2 Lactation
Risk Summary
It is not known whether PULMOZYME is present in human milk. In a pharmacokinetic study in Cynomolgus monkeys, levels of dornase alfa detected in milk were less than 0.1% of the maternal serum concentration at 24 hours after dosing [intravenous bolus dose (0.1 mg/kg) of dornase alfa followed by an intravenous infusion (0.080 mg/kg/hr) over a 6-hour period] on post-partum day 14. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for PULMOZYME and any potential adverse effects on the breastfed child from PULMOZYME or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of PULMOZYME in conjunction with standard therapies for cystic fibrosis have been established in pediatric patients. Use of PULMOZYME in pediatric patients is supported by evidence in the following age groups:
- Patients 5 to 17 years of age: Use of PULMOZYME in patients 5 to 17 years of age is supported by evidence from a randomized, placebo-controlled trial of 303 of clinically stable cystic fibrosis patients 5 to 17 years of age who received PULMOZYME [see Clinical Studies (14)].
- Patients less than 5 years: Use of PULMOZYME in patients less than 5 years of age is supported by extrapolation of efficacy data in patients 5 years of age and older with additional safety data in 65 pediatric patients aged 3 months to less than 5 years who received PULMOZYME 2.5 mg daily by inhalation for 2 weeks [see Adverse Reactions (6.1) and Clinical Studies (14)].
8.5 Geriatric Use
Cystic fibrosis is primarily a disease of children and young adults. Clinical studies of PULMOZYME did not include sufficient numbers of subjects aged 65 or older to determine whether they respond differently from younger subjects.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
PULMOZYME is recombinant human deoxyribonuclease I (rhDNase), an enzyme which selectively cleaves DNA. In preclinical in vitro studies, PULMOZYME hydrolyzes the DNA in sputum of CF patients and reduces sputum viscoelasticity. In CF patients, retention of viscous purulent secretions in the airways contributes both to reduced pulmonary function and to exacerbations of infection. Purulent pulmonary secretions contain very high concentrations of extracellular DNA released by degenerating leukocytes that accumulate in response to infection.
12.3 Pharmacokinetics
When 2.5 mg PULMOZYME was administered by inhalation to eighteen CF patients, mean sputum concentrations of 3 µg/mL DNase were measurable within 15 minutes. Mean sputum concentrations declined to an average of 0.6 µg/mL two hours following inhalation. Inhalation of up to 10 mg TID of PULMOZYME by 4 CF patients for six consecutive days, did not result in a significant elevation of serum concentrations of DNase above normal endogenous levels. After administration of up to 2.5 mg of PULMOZYME twice daily for six months to 321 CF patients, no accumulation of serum DNase was noted. Dornase alfa is expected to be metabolized by proteases present in biological fluids. A human intravenous dose study suggested an elimination half-life of 3-4 hours for dornase alfa.
PULMOZYME, 2.5 mg by inhalation, was administered daily to 98 patients aged 3 months to ≤ 10 years, and bronchoalveolar lavage (BAL) fluid was obtained within 90 minutes of the first dose. BAL DNase concentrations were detectable in all patients but showed a broad range, from 0.007 to 1.8 µg/mL. Over an average of 14 days of exposure, serum DNase concentrations (mean ± s.d.) increased by 1.1 ± 1.6 ng/mL for the 3 months to < 5 year age group and by 0.8 ± 1.2 ng/mL for the 5 to ≤ 10 year age group. The relationship between BAL or serum DNase concentration and adverse experiences and clinical outcomes is unknown.
SPL UNCLASSIFIED SECTION
Pulmozyme**®**** (dornase alfa)**
Inhalation Solution
Manufactured by:
Genentech, Inc.
A Member of the Roche Group
1 DNA Way
South San Francisco, CA 94080-4990
US License No. 1048
©2024 Genentech, Inc. All rights reserved
©2024 Genentech, Inc.
Pulmozyme® is a registered trademark of Genentech, Inc.
INSTRUCTIONS FOR USE SECTION
INSTRUCTIONS FOR USE
PULMOZYME**®**** (PULL-muh-zyme)**
(dornase alfa)
Inhalation Solution
This Instructions for Use contains information on how to use PULMOZYME with the following recommended vibrating mesh nebulizers:
|
Recommended Vibrating Mesh Nebulizers |
|---|
|
eRapid® Nebulizer System |
|
Innospire Go |
|
Pulmogine Vibrating Mesh Nebulizer |
|
AireHealth Nebulizer™ |
|
Intelligent Mesh Nebulizer |
See the other side of this Instructions for Use for****information on use with Jet Nebulizers and Compressors
Read and understand this Instructions for Use and the nebulizer manufacturer's instruction manual before you start taking Pulmozyme and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
This information does not take the place of the manufacturer's instruction manual for the vibrating mesh nebulizer.
The vibrating mesh nebulizer changes the Pulmozyme liquid medicine into a fine mist you inhale by breathing through a mouthpiece.
Do not use any other inhaled medicines in the nebulizer at the same time. Keep all other inhaled medication systems completely separate from Pulmozyme.
The eRapid Nebulizer System should only be used by adults and children who can use a mouthpiece, and not by younger children who need a mask to take Pulmozyme.
Follow the instructions on this side of the Instructions for Use to give Pulmozyme using a vibrating mesh nebulizer.
Important Information You Need to Know Before Using PULMOZYME
Read and follow the nebulizer manufacturer's instruction manual for correct use and maintenance:
- to clean the nebulizer before first use and after each use as recommended
- to disinfect the nebulizer parts by using the disinfecting method recommended
- to replace nebulizer parts as recommended
Supplies you will need to give a dose of PULMOZYME:
1 Pulmozyme ampule*(SeeFigure A)**
- Vibrating mesh nebulizer and its parts
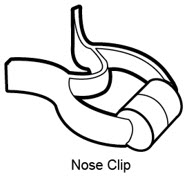
- Manufacturer's instruction manual for the vibrating mesh nebulizer Nose clip (optional) (See**Figure B***)**
|
|
|
Pulmozyme ampule |
|
|
|
Figure B |
|
Prepare the vibrating mesh nebulizer:
|
|
|
Figure C | |
|
Step 2. Gather the nebulizer. Make sure you have all parts and make sure they are clean and not damaged. Prepare and test it as recommended in the manufacturer's instruction manual.
| |
|
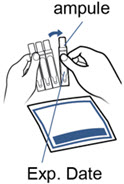
Step 3. Gather the Pulmozyme ampule and check the expiration date.
|
|
|
Step 4. Check the Pulmozyme ampule.
|
|
|
Step 5. Put the vibrating mesh nebulizer together.
| |
|
Step 6. Prepare to take the Pulmozyme treatment.
Taking your dose of Pulmozyme with a nebulizer:
|
|
|
Step 7. Breathing through the mouthpiece. *Skip to Step 8 if you are using a facemask to take your dose of Pulmozyme.
| |
|
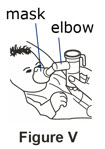
Step 8. Breathing through the facemask.
| |
|
After Your Treatment with Pulmozyme:
|
How should I store Pulmozyme?
*Store Pulmozyme ampules at a refrigerated temperature between 36°F to 46°F (2°C to 8°C) in their protective foil pouch to protect from light and heat until you are ready to use them. When the protective foil pouch is opened, the unused ampules must be kept refrigerated in the protective foil pouch to protect from light and heat.
- When traveling, Pulmozyme ampules should be kept refrigerated in their protective foil pouch to protect from light and heat.
- Protect Pulmozyme from excessive heat and light. *Do not use Pulmozyme if the ampules have been exposed to room temperature at 72°F to 82°F (22°C to 28°C) for more than a total of60 hours or if the solution has turned cloudy or discolored. *Do not use Pulmozyme past the expiration date printed on the ampule.
Genentech, Inc.
A Member of the Roche Group
1 DNA Way
South San Francisco, CA 94080-4990
US License No. 1048
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 02/2024
Representative sample of labeling (see the HOW SUPPLIED section for complete listing):
DESCRIPTION SECTION
11 DESCRIPTION
Dornase alfa is a recombinant human deoxyribonuclease I (rhDNase) an enzyme which selectively cleaves DNA. The protein is produced by genetically engineered Chinese Hamster Ovary (CHO) cells containing DNA encoding for the native human protein, deoxyribonuclease I (DNase). The product is purified by column chromatography and tangential flow filtration. The purified glycoprotein contains 260 amino acids with an approximate molecular weight of 37,000 daltons. The primary amino acid sequence is identical to that of the native human enzyme.
PULMOZYME (dornase alfa) inhalation solution is administered by inhalation of an aerosol mist produced by a compressed air driven nebulizer or a recommended nebulizer system [see Clinical Studies (14) and Dosage and Administration (2.2)]. PULMOZYME is a sterile, clear, colorless, highly purified solution in single-dose ampules. Each ampule delivers 2.5 mL of the solution to the nebulizer bowl. Each mL of aqueous solution contains 1 mg dornase alfa, calcium chloride dihydrate (0.15 mg) and sodium chloride (8.77 mg). The solution contains no preservative. The nominal pH of the solution is 6.3.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
PULMOZYME produced no treatment-related increases in the incidence of tumors in a lifetime study in Sprague Dawley rats that were administered inhaled doses up to 0.246 mg/kg/day (approximately 30 times the MRHD in adults). There was no increase in the development of benign or malignant neoplasms and no occurrence of unusual tumor types in rats after lifetime exposure.
PULMOZYME tested negative in the following genotoxicity assays: the in vitro Ames assay, in vitro mouse lymphoma assay, and in vivo mouse bone marrow micronucleus assay. No evidence of impairment of fertility was observed in male and female rats that received intravenous doses up to 10 mg/kg/day (approximately 600 times the MRHD in adults).
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
Trial in CF Patients with FVC ≥40% of Predicted
PULMOZYME has been evaluated in a randomized, placebo-controlled trial of clinically stable cystic fibrosis patients, 5 years of age and older, with baseline forced vital capacity (FVC) greater than or equal to 40% of predicted and receiving standard therapies for cystic fibrosis. Patients were treated with placebo (325 patients), 2.5 mg of PULMOZYME once a day (322 patients), or 2.5 mg of PULMOZYME twice a day (321 patients) for six months administered via a Hudson T Up-draft II® nebulizer with a Pulmo-Aide® compressor.
Both doses of PULMOZYME resulted in significant reductions in the number of patients experiencing respiratory tract infections requiring use of parenteral antibiotics compared with the placebo group. Administration of PULMOZYME reduced the relative risk of developing a respiratory tract infection by 27% and 29% for the 2.5 mg daily dose and the 2.5 mg twice daily dose, respectively (see Table 3). The data suggest that the effects of PULMOZYME on respiratory tract infections in older patients ( > 21 years) may be smaller than in younger patients, and that twice daily dosing may be required in the older patients. Patients with baseline FVC > 85% may also benefit from twice a day dosing (see Table 3). The reduced risk of respiratory infection observed in PULMOZYME treated patients did not directly correlate with improvement in FEV1 during the initial two weeks of therapy.
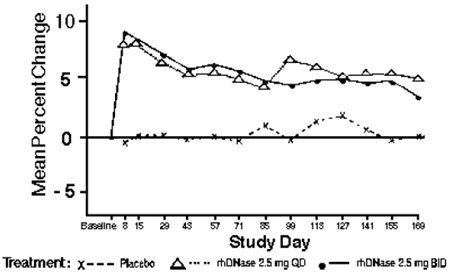
Within 8 days of the start of treatment with PULMOZYME, mean FEV1 increased 7.9% in those treated once a day and 9.0% in those treated twice a day compared to the baseline values. The overall mean FEV1 during long-term therapy increased 5.8% from baseline at the 2.5 mg daily dose level and 5.6% from baseline at the 2.5 mg twice daily dose level. Placebo recipients did not show significant mean changes in pulmonary function testing (see Figure 1).
For patients 5 years of age or older, with baseline FVC greater than or equal to 40%, administration of PULMOZYME decreased the incidence of occurrence of first respiratory tract infection requiring parenteral antibiotics, and improved mean FEV1, regardless of age or baseline FVC.
Table 3. Incidence of First Respiratory Tract Infection Requiring Parenteral Antibiotics in Patients with FVC ≥40% of Predicted|
Placebo |
2.5 mg QD |
2.5 mg BID | |
|
Percent of Patients Infected |
43% |
34% |
33% |
|
Relative Risk (vs placebo) |
0.73 |
0.71 | |
|
p-value (vs placebo) |
0.015 |
0.007 | |
|
Subgroup by Age and Baseline FVC |
Placebo |
2.5 mg QD |
2.5 mg BID |
|
Age | |||
|
5-20 years |
42% (201) |
25% (199) |
28% (184) |
|
21 years and older |
44% (124) |
48% (123) |
39% (137) |
|
Baseline FVC | |||
|
40-85% Predicted |
54% (194) |
41% (201) |
44% (203) |
|
27% (131) |
21% (121) |
14% (118) |
|
|
Trial in CF Patients with FVC <40% of Predicted
PULMOZYME has also been evaluated in a second randomized, placebo-controlled trial in clinically stable patients with baseline FVC < 40% of predicted. Patients were enrolled and treated with placebo (162 patients) or PULMOZYME 2.5 mg QD (158 patients) for twelve weeks. In patients who received PULMOZYME, there was an increase in mean change (as percent of baseline) compared to placebo in FEV1 (9.4% vs. 2.1%, p < 0.001) and in FVC (12.4% vs. 7.3%, p < 0.01). PULMOZYME did not significantly reduce the risk of developing a respiratory tract infection requiring parenteral antibiotics (54% of PULMOZYME patients vs. 55% of placebo patients had experienced a respiratory tract infection by 12 weeks, relative risk = .93, p = 0.62).
The effect of PULMOZYME on exercise tolerance has not been established in adult and pediatric patients.
Other Studies
Clinical trials have indicated that PULMOZYME therapy can be continued or initiated during an acute respiratory exacerbation.
Short-term dose ranging studies demonstrated that doses in excess of 2.5 mg BID did not provide further improvement in FEV1. Patients who have received drug on a cyclical regimen (i.e., administration of PULMOZYME 10 mg BID for 14 days, followed by a 14 day wash out period) showed rapid improvement in FEV1 with the initiation of each cycle and a return to baseline with each PULMOZYME withdrawal.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
PULMOZYME (dornase alfa) inhalation solution is a sterile, clear, colorless solution supplied in:
- 30 unit cartons containing 5 foil pouches of 6 single-dose ampules. Each 2.5 mL ampule contains 2.5 mg of dornase alfa (1 mg/mL): NDC 50242-100-40.
Storage and Handling
Store PULMOZYME ampules at a refrigerated temperature between 2°C to 8°C (36°F to 46°F) in their protective foil to protect from light and heat. Once the protective foil pouch is opened, the unused ampules must be kept refrigerated in the protective foil pouch to protect from light and heat. Do not use beyond the expiration date stamped on the ampule. During transport, keep the ampules refrigerated in their protective foil pouch to protect from light and heat. Do not use if the ampules are exposed to room temperature (22°C to 28°C [72°F to 82°F]) for more than a total of 60 hours. Avoid excessive heat and light.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise patients to read the FDA-approved patient labeling (Instructions for Use).
Preparation
Advise patients to squeeze each ampule prior to use in order to check for leaks. The solution should be discarded if it is cloudy or discolored. Once opened, the entire contents of the ampule must be used or discarded [see Dosage and Administration (2.2)].
Drug Incompatibilities
Instruct patients not to dilute or mix PULMOZYME with other drugs in the nebulizer. Mixing of PULMOZYME with other drugs could lead to adverse physicochemical and/or functional changes in PULMOZYME or the admixed compound [see Dosage and Administration (2.2)].
Storage
Instruct patients on the proper techniques to store and handle PULMOZYME [see How Supplied/Storage and Handling (16)].
Manufacturer's Instruction Manual
Instruct patients to read and follow the manufacturer's instruction manual for the proper use and maintenance of the jet nebulizer/compressor system, or the vibrating mesh nebulizer used in PULMOZYME delivery.
SPL PATIENT PACKAGE INSERT SECTION
INSTRUCTIONS FOR USE
PULMOZYME**®****(PULL-muh-zyme)**
(dornase alfa)
Inhalation Solution
This Instructions for Use contains information on how to use PULMOZYME with Jet Nebulizers and Compressors
See the other side of this Instructions for Use for****information on use of Pulmozyme with the recommended vibrating mesh nebulizers
Read and understand this Instructions for Use and the nebulizer manufacturer's instruction manual before you start taking Pulmozyme and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or your treatment.
A nebulizer and a compressor are used together to give a dose of Pulmozyme. A nebulizer changes the Pulmozyme liquid medicine into a fine mist you inhale by breathing through a mouthpiece. A compressor gives the nebulizer power and makes the nebulizer work.
Pulmozyme should only be used with the approved nebulizers and appropriate compressors asrecommended below, or with the recommended vibrating mesh nebulizers (see other side of this Instructions for Use). Read and follow the manufacturer's instruction manual.
Do not use any other inhaled medicines in the nebulizer at the same time. Keep all other inhaled medicine systems completely separate from Pulmozyme.
Use the mouthpiece or face mask provided with the nebulizer kit.
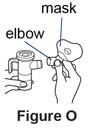
If your child cannot breathe in or breathe out by mouth, you may use the PARI BABY reusable nebulizer, but you should discuss it with your doctor first. The PARI BABY nebulizer is the same as the PARI LC Plus Jet system, except the mouthpiece is replaced by a tight-fitting face mask connected to an elbow piece.
Follow the steps on this side of the Instructions for Use to give Pulmozyme using the following jet nebulizer systems
|
Jet Nebulizer |
Compressor |
|---|---|
|
Hudson T Up-draft II |
A compressor with the following specifications is recommended:
|
|
Marquest Acorn II | |
|
PARI LC Plus | |
|
PARI BABY | |
|
Durable Sidestream |
For additional information on an appropriate compressor to use with Pulmozyme, read the manufacturer's instruction manual for the recommended nebulizer.
Important Information You Need to Know Before Using PULMOZYME
Read and follow the nebulizer manufacturer's instruction manual for correct use and maintenance:
- to clean the nebulizer before first use and after each use as recommended.
- to disinfect the nebulizer parts by using the disinfecting method recommended.
- to replace nebulizer parts as recommended.
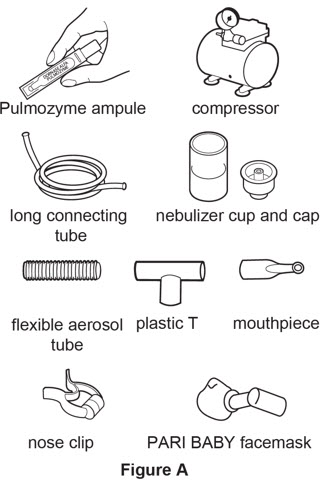
Supplies you will need to give a dose of Pulmozyme (SeeFigure A):
- 1 Pulmozyme ampule
- Compressor
- Nebulizer cup and cap (screw-on or snap-on)
- Plastic T (not needed for Sidestream nebulizer or PARI BABY)
- Flexible aerosol tube (not needed for Sidestream nebulizer or PARI BABY)
- Mouthpiece (clean) or PARI BABY facemask
- Long connecting tube
- Nose clip (optional, not needed for PARI BABY)
|
Preparing the jet nebulizer and compressor:
|
|
|
Step 2. Gather the nebulizer and test the compressor.
|
|
|
Step 3. Gather the Pulmozyme ampule and check the expiration date.
|
|
|
Step 4. Check the Pulmozyme ampule.
|
|
|
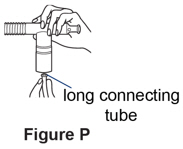
Step 5. Attach the long connecting tube to the compressor.
|
|
|
Step 6. Attach the mouthpiece. *Skip to Step 7 if you use the Sidestream nebulizer or the PARI BABY nebulizer.
|
|
|
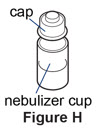
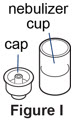
Step 7. Remove the cap from the cup.
|
|
|
|
|
Step 8. Open the Pulmozyme ampule.
|
|
|
Step 9. Pour the full Pulmozyme dose into the nebulizer cup.
|
|
|
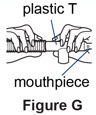
Step 10. Connect the plastic T.
|
|
|
If you are using the Sidestream nebulizer, attach the mouthpiece to the top of the nebulizer (See**Figure N***).** |
|
|
If you are using the PARI BABY nebulizer, connect the elbow piece and mask to the nebulizer outlet (See**Figure O***).** |
|
|
Step 11. Attach the long connecting tube to the cup.
|
|
|
Step 12. Turn on the compressor.
|
|
|
Taking your dose of Pulmozyme with a nebulizer: | |
|
Step 13. Breathe through the mouthpiece. *Skip to Step 14 if you are using the PARI BABY to give Pulmozyme to your child.
|
|
|
|
|
If you are using the PARI BABY nebulizer to give Pulmozyme to your child,
follow the instructions below in Step 14. If not, go to Step 15 | |
|
|
|
|
|
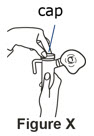
It is important that your child inhale the full dose of Pulmozyme. If you find a leak or feel moisture coming from the nebulizer during the treatment, turn off the compressor and make sure the nebulizer cap is sealed correctly before starting the compressor again**(SeeFigure X)**. |
|
|
After your treatment with Pulmozyme:
| |
|
How should I store Pulmozyme? *Store Pulmozyme ampules at a refrigerated temperature between 36°F to 46°F (2°C to 8°C) in their protective foil pouch to protect from light and heat until you are ready to use them. When the protective foil pouch is opened, the unused ampules must be kept refrigerated in the protective foil pouch to protect from light and heat.
|
Genentech, Inc.
A Member of the Roche Group
1 DNA Way
South San Francisco, CA 94080-4990
US License No. 1048
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 02/2024