Spinosad
These highlights do not include all the information needed to use Spinosad Topical Suspension safely and effectively. See full prescribing information for Spinosad Topical Suspension. Spinosad Topical Suspension Initial U.S. Approval: 2011
8e87bd3b-db25-4e97-bf57-96c875f67ce1
HUMAN PRESCRIPTION DRUG LABEL
May 31, 2023
Allegis Pharmaceuticals, LLC
DUNS: 792272861
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
spinosad
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
Drug Labeling Information
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
1.1 Head Lice Infestations
Spinosad Topical Suspension is indicated for the topical treatment of head lice infestations in adult and pediatric patients 6 months of age and older.
Adjunctive Measures for Head Lice Infestations
Spinosad Topical Suspension should be used in the context of an overall lice management program:
- Wash in hot water or dry-clean all recently worn clothing, hats, used bedding and towels.
- Wash personal care items such as combs, brushes and hair clips in hot water.
- A fine-tooth comb or special nit comb may be used to remove dead lice and nits.
1.2 Scabies Infestations
Spinosad Topical Suspension is indicated for the topical treatment of scabies infestations in adult and pediatric patients 4 years of age and older.
Adjunctive Measures for Scabies Infestations
- Wash in hot water or dry-clean any bedding, clothing and towels used by anyone having scabies.
Spinosad Topical Suspension is a pediculicide indicated for the topical treatment of head lice infestations in adult and pediatric patients 6 months of age and older. ( 1.1)
Spinosad Topical Suspension is a scabicide indicated for the topical treatment of scabies infestations in adult and pediatric patients 4 years of age and older, ( 1.2)
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
|
Indications and Usage ( 1) |
04/2021 |
|
Dosage and Administration ( 2) |
04/2021 |
|
Warnings and Precautions ( 5) |
04/2021 |
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
- For topical use only. Spinosad Topical Suspension is not for oral, ophthalmic, or intravaginal use.
- Avoid contact with eyes. If Spinosad Topical Suspension gets in or near the eyes, rinse thoroughly with water.
2.2 Treatment of Head Lice Infestations
- Shake bottle well.
- Apply a sufficient amount of Spinosad Topical Suspension to cover dry scalp, then apply to dry hair. Depending on hair length, apply up to 120 mL (one bottle) to adequately cover scalp and hair.
- Leave on for 10 minutes, then thoroughly rinse off with warm water.
- Wash hands after use.
- If live lice are seen 7 days after the first treatment, a second treatment should be applied.
- Apply Spinosad Topical Suspension on pediatric patient only under direct supervision of an adult [see Warnings and Precautions (5.1)].
2.3 Treatment of Scabies Infestations
- Shake bottle well.
- Apply a sufficient amount of Spinosad Topical Suspension to skin to completely cover the body from the neck to the toes (including the soles of the feet).
- For patients with balding scalp, also apply product to the scalp, hairline, temples, and forehead.
- Allow to absorb into the skin and dry for 10 minutes before getting dressed.
- Leave on the skin for at least 6 hours before showering or bathing.
- Apply Spinosad Topical Suspension on pediatric patient only under direct supervision of an adult .
- For topical use only. Not for oral, ophthalmic, or intravaginal use. ( 2)
- Treatment of head lice infestations ( 2.2):
- Shake bottle well
- Apply a sufficient amount to cover dry scalp, then apply to dry hair
- Rinse off with warm water after 10 minutes
- Repeat treatment only if live lice are seen 7 days after first treatment
- Treatment of scabies infestations ( 2.3):
- Shake bottle well
- Apply product to skin by rubbing it in to completely cover the body from the neck down to the soles of the feet
- Patients with balding scalp should also apply product to the scalp, hairline, temples, and forehead
- Allow to absorb in the skin and dry for 10 minutes before getting dressed
- Leave on the skin for at least 6 hours before showering or bathing
DESCRIPTION SECTION
11 DESCRIPTION
Spinosad Topical Suspension is a slightly opaque, light orange-colored, viscous topical suspension.
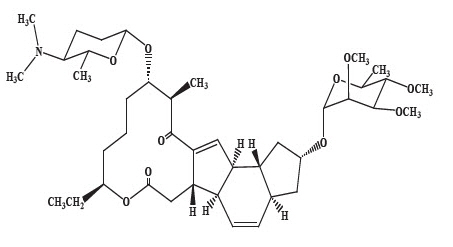
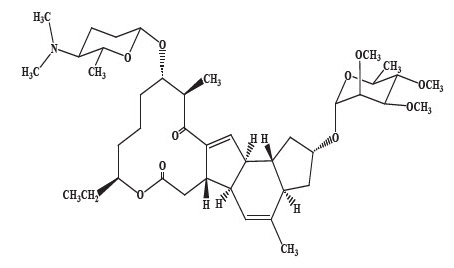
Spinosad Topical Suspension is a pediculicide and scabicide. Spinosad, the active ingredient, is derived from the fermentation of a soil actinomycete bacterium, Saccharopolyspora spinosa.
Spinosad is a mixture of spinosyn A and spinosyn D in a ratio of approximately 5 to 1 (spinosyn A to spinosyn D).
Spinosyn A: The chemical name is: 1 H-as- Indaceno[3,2-d]oxacyclododecin-7,a5-dione, 2-[(6-deoxy-2,3,4-tri-O-methyl- alpha-L-mannopyranosyl)oxy]-13-[[2R,5S,6R)-5-(dimethylamino) tetrahydro-6-methyl-2 H-pyran-2-yl]oxy]-9-ethyl-2,3,3a,5a,5b,6,9,10,11,12,13,14,16a,16b-tetradecahydro-14-methyl-, (2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-
Spinosyn D: The chemical name is: 1 H-as- Indaceno[3,2-d]oxacyclododecin-7,15-dione, 2-[(6-deoxy-2,3,4-tri-O-methyl- alpha-L-mannopyranosyl)oxy]-13-[[2R,5S,6R)-5-(dimethylamino) tetrahydro-6-methyl-2 H-pyran-2-yl]oxy]-9-ethyl-2,3,3a,5a,5b,6,9,10,11,12,13,14,16a,16b-tetradecahydro-4,14-dimethyl-, (2S,3aSR,5aS,5bS,9S,13S,14R,16aS,16bS)-
|
|
|
|
Spinosyn A (C41H65NO10) |
Spinosyn D (C42H67NO10) |
Spinosad Topical Suspension contains 9 mg spinosad per gram in a vehicle consisting of Benzyl Alcohol, Butylated Hydroxytoluene, Ceteareth-20, Cetearyl Alcohol, FD&C Yellow #6, Hexylene Glycol, Hydroxyethyl Cellulose, Isopropyl Alcohol, Propylene Glycol, Stearalkonium Chloride, Water, Hydrochloric acid (HCl) as pH adjuster.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Spinosad causes neuronal excitation in insects. After periods of hyperexcitation, lice and mites become paralyzed and die.
12.2 Pharmacodynamics
The pharmacodynamics of Spinosad Topical Suspension has not been studied.
12.3 Pharmacokinetics
Head Lice Infestations
An open-label, single-center trial was conducted over a period of seven days to determine the pharmacokinetic profile of spinosad 1.8% in pediatric subjects with head lice infestation. Fourteen (14) subjects, 4 – 15 years of age, with head lice were enrolled into the trial. All subjects applied a single topical (scalp) treatment of spinosad 1.8% for 10 minutes, after which the test article was washed off, and subjects underwent plasma sampling. Results demonstrated that spinosad was below the limit of quantitation (3 ng/mL) in all samples. Plasma concentration of benzyl alcohol was not determined in these subjects.
An open-label, two-center trial was conducted over a period of 23 days to determine the pharmacokinetic profile of spinosad 0.9% and the ingredient benzyl alcohol in pediatric subjects with a head lice infestation. Twenty-six (26) subjects between 6 months to 4 years of age were enrolled into the study per protocol. All subjects applied a single topical (scalp) treatment of spinosad 0.9% for 10 minutes, after which the test article was washed off, and subjects underwent plasma sampling over a 12 hour period. Plasma spinosad concentrations were below the limit of quantitation (3 ng/mL) in all samples.
Benzyl alcohol was quantifiable (above 1 μg/mL) in a total of 8 plasma samples in 6 out of 26 subjects (25%): four out of 12 subjects in the 6 months to <2 years age group and two out of 14 subjects in the 2 to 4 years age group. The highest observed concentration was 2.37 μg/mL. Benzyl alcohol concentrations at 12 hours post-treatment were below limit of quantification (1 μg/mL) for all subjects.
Scabies Infestations
An open-label multi-center trial was conducted to determine the pharmacokinetic profile of spinosad 0.9% and the ingredient benzyl alcohol in pediatric subjects with scabies infestation. The PK bioavailability study was completed in 19 pediatrics subjects 5 to 16 years of age. All subjects applied a single topical body treatment of spinosad 0.9% from the neck down to the soles of the feet and allowed treatment to remain on the body for a minimum of 6 hours after which the test article was washed off. Subjects underwent plasma sampling over a 12-hour period after treatment. Plasma spinosad concentrations were below the limit of quantification (3 ng/mL) in all samples.
Benzyl alcohol was quantifiable (above 1 μg/mL) in a total of 9 plasma samples in 6 out of 19 subjects (32%): in 3 out of 10 subjects in the 5 to 9 year age group and in 3 out of 9 subjects in the 10 to 16 years age group. The highest observed concentration was 3.94 μg/mL at 0.5 hours post-treatment but was below limit of quantification at 1 hour post-treatment for one subject in the 10 to 16 years age group. There were two subjects with a benzyl alcohol concentration at 3 hours post-treatment with the highest value of 3.53 μg/mL for one subject in the 5 to 9 years age group. Plasma concentrations were below limit of quantification (1 μg/mL) at 3 hours for all other subjects; no subject had measurable concentrations at the 6 and12 hour time points. The mean (SD) Cmax, Tmax, and AUC0-12h values for benzyl alcohol were 2.737 (1.107) μg/mL, 1.42 (1.242) hours, and 19.240 μg∙h/mL, respectively.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise patients to read the FDA-approved patient labeling (Patient Information)
Important Administration Instructions
Instruct the patient to:
- Shake bottle well immediately prior to application.
- Do not swallow.
- Avoid contact with eyes. If Spinosad Topical Suspension gets in or near the eyes, rinse thoroughly with water.
- Apply Spinosad Topical Suspension on pediatric patient only under direct supervision of an adult.
- If skin/scalp irritation occurs after use, contact your physician [see Adverse Reactions (6.1)] .
Head Lice Infestation Treatment
- Apply Spinosad Topical Suspension only on dry scalp and dry scalp hair.
- Repeat treatment only if live lice are seen seven (7) days after first treatment.
- Wash hands after applying Spinosad Topical Suspension.
Scabies Infestation Treatment
- Apply Spinosad Topical Suspension to completely cover the body from the neck to the toes (including the soles of the feet).
- For patients with balding scalp, also apply product to the scalp, hairline, temples, and forehead.
- Allow it to absorb in the skin and dry for 10 minutes before getting dressed.
- Leave on the skin for at least 6 hours before showering or bathing.
- Wash your hands after applying Spinosad Topical Suspension to someone else.
- For breastfeeding women, remove Spinosad Topical Suspension from the breast with soap and water before breastfeeding to avoid direct infant exposure to Spinosad Topical Suspension [see Use in Specific Populations (8.2)]